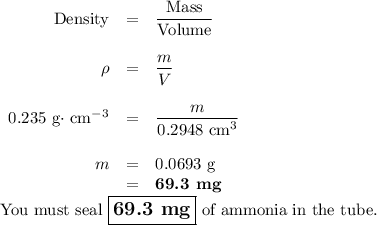
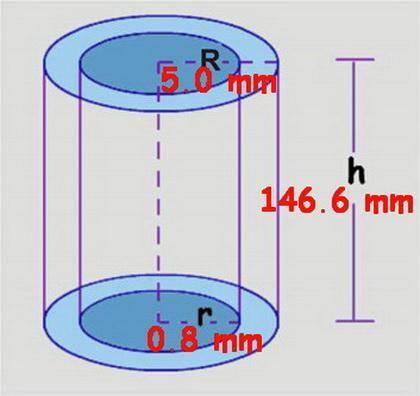
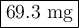At its critical point, ammonia has a density of 0.235 g cm23. You have a special thickwalled glass tube that has a 10.0mm outside diameter, a wall thickness of 4.20 mm, and a length of 155 mm. How much ammonia must you seal into the tube if you wish to observe the disappearance of the meniscus as you heat the tube and its contents to a temperature higher than 132.23°C, the critical temperature?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 20:00
If one fission reaction of a uranium-235 atom produced two neutrons, how many neutrons would be released if the chain reaction occurred three more times?
Answers: 1


Chemistry, 23.06.2019 01:00
Heat energy, carbon dioxide, and water are released through which process? a. photosynthesis b. depolymerization c. digestion d. cellular respiration
Answers: 1

Chemistry, 23.06.2019 01:10
Volume is a measurement of how fast particles of a substance are moving
Answers: 3
You know the right answer?
At its critical point, ammonia has a density of 0.235 g cm23. You have a special thickwalled glass...
Questions





English, 26.10.2019 10:43




Mathematics, 26.10.2019 10:43


Arts, 26.10.2019 10:43

Mathematics, 26.10.2019 10:43

Mathematics, 26.10.2019 10:43



Mathematics, 26.10.2019 10:43

English, 26.10.2019 10:43


History, 26.10.2019 10:43