
Chemistry, 24.02.2020 21:27 anitadefrances
A metal has a body-centered cubic lattice with a unit cell edge length of 2.866 Å (1 Å = 10⁻¹⁰ m). The density of the metal is 7.87 g/cm³. What is the mass of an atom of this metal? (1 m = 10¹² pm).

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:10
What does a particular point on a line of a phase diagram represent? o a. the maximum temperature a substance can exist at without bonds breaking b. the pressure created by the kinetic energy of molecules at a particular temperature c. the melting point or boiling point of a substance at a specific pressure d. the conditions in which temperature and pressure have equal effects on a substance
Answers: 2

Chemistry, 22.06.2019 06:30
Type the correct answer in the box. spell all words correctly.what is the correct term for living the most sustainable life you can within your current circumstances? when your are being as sustainable as you can within your current lifestyle, you are said to be sustainability.
Answers: 3

Chemistry, 22.06.2019 12:00
What is the subscript for oxygen in its molecular formula
Answers: 1

Chemistry, 22.06.2019 12:30
In france, grapes are 1.95 euros per kilogram. what is the cost of grapes, in dollars per pound, if the exchange rate is 1.14 dollars/euro? (2.6)
Answers: 3
You know the right answer?
A metal has a body-centered cubic lattice with a unit cell edge length of 2.866 Å (1 Å = 10⁻¹⁰ m). T...
Questions


History, 03.03.2022 14:00




Mathematics, 03.03.2022 14:00

Business, 03.03.2022 14:00

Chemistry, 03.03.2022 14:00


Social Studies, 03.03.2022 14:00

Chemistry, 03.03.2022 14:00



Mathematics, 03.03.2022 14:00

SAT, 03.03.2022 14:00

Chemistry, 03.03.2022 14:00


Biology, 03.03.2022 14:00


SAT, 03.03.2022 14:00

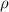 of a unit cell can be computed as:
of a unit cell can be computed as: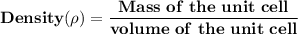
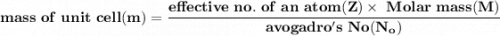 Volume of the unit cell = a³
Volume of the unit cell = a³ 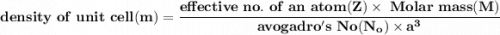
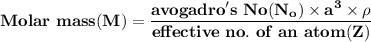
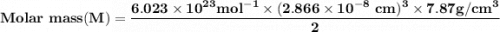
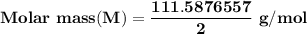
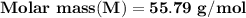
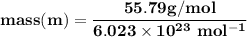
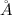
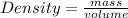
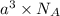
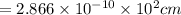
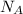 =Avogadro Number =6.023×10²³
=Avogadro Number =6.023×10²³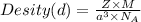
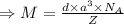
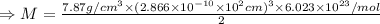
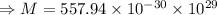 g/mol
g/mol

