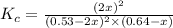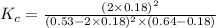Consider the reaction between NONO and Cl2Cl2 to form NOClNOCl: 2NO(g)+Cl2(g)⇌2NOCl(g)2NO(g)+Cl2(g) ⇌2NOCl(g) A reaction mixture at a certain temperature initially contains only [NO]=[NO]= 0.53 MM and [Cl2]=[Cl2]= 0.64 MM. After the reaction comes to equilibrium, the concentration of NOClNOCl is 0.36 MM. Part A Find the value of the equilibrium constant (Kc)(Kc) at this temperature.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:00
Which statement best explains the relationship between an area is geography and the temperature of its surface water
Answers: 1

Chemistry, 22.06.2019 22:30
Molecular iodine, i2(g), dissociates into iodine atoms at 625 k with a first-order rate constant of 0.271 s−1. part a part complete what is the half-life for this reaction?
Answers: 3

Chemistry, 22.06.2019 23:00
Which of your 24 wells had indications that a chemical reaction occurred? how were you able to tell that a chemical reaction occurred? which of your 24 wells had indications that a physical reaction occurred? how were you able to tell that a physical reaction occurred? report on both mixing and evaporation. make a general statement about whether your hypotheses were validated or rejected. must your hypotheses be correct for this to be a successful laboratory?
Answers: 3

Chemistry, 23.06.2019 01:30
How does the attraction between particles affect the ability of a solvent to dissolve in a substance
Answers: 1
You know the right answer?
Consider the reaction between NONO and Cl2Cl2 to form NOClNOCl: 2NO(g)+Cl2(g)⇌2NOCl(g)2NO(g)+Cl2(g)...
Questions

History, 26.08.2019 12:00

Social Studies, 26.08.2019 12:00



Chemistry, 26.08.2019 12:00

Computers and Technology, 26.08.2019 12:00

Geography, 26.08.2019 12:00

Health, 26.08.2019 12:00


History, 26.08.2019 12:00





English, 26.08.2019 12:00

History, 26.08.2019 12:00



English, 26.08.2019 12:00

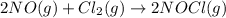
![K_c=\frac{[NOCl]^2}{[NO]^2[Cl_2]}](/tpl/images/0521/6978/56950.png)