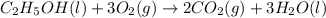Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 16:20
What would you do if you told the guy you liked that you liked him
Answers: 1

Chemistry, 22.06.2019 01:00
Which of the following is always a reactant in a combustion reaction? oxygen nitrogen hydrogen carbon
Answers: 1

Chemistry, 22.06.2019 16:50
What is conserved in the reaction shown below? h2(g) + cl2 (g) --> 2hcl(g)a. mass onlyb. mass and moles onlyc. mass, moles, and molecules onlyd. mass, moles, molecules, and volume
Answers: 2

Chemistry, 22.06.2019 18:20
Which reason best explains why metals are malleable? a)because they have delocalized electrons b)because they have localized electrons c)because they have ionic bonds d)because they have rigid bonds
Answers: 2
You know the right answer?
Starting with the partially balanced equation, C2H5OH(l)+?O2(g)→2CO2(g)+3H2O(l) what coefficient sho...
Questions


Social Studies, 05.12.2020 09:20



Mathematics, 05.12.2020 09:20


English, 05.12.2020 09:20



Physics, 05.12.2020 09:20



Health, 05.12.2020 09:20

Mathematics, 05.12.2020 09:20



Mathematics, 05.12.2020 09:20

Chemistry, 05.12.2020 09:20

Business, 05.12.2020 09:20

Mathematics, 05.12.2020 09:20