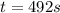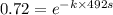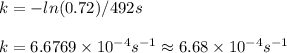Chemistry, 24.02.2020 18:41 kprincess16r
The isomerization reaction CH3NC → CH3CN obeys the first-order rate law, rate = k[CH3NC], in the presence of an excess of argon. Measurement at 500. K reveals that in 492 seconds, the concentration of CH3NC has decreased to 72% of its original value. Calculate the rate constant (k) of the reaction at 500. K.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 15:30
Becquerel expected to find ( he developed the photographic plate that had sun-exposed minerals on top of it. becquerel expected to find ( he developed the photographic plate that had been in the closed drawer.
Answers: 2

Chemistry, 22.06.2019 10:30
Apiece of metal with a length of 1.42 cm was measured using four different instruments. which of the following measurements is the most accurate?
Answers: 3

Chemistry, 22.06.2019 16:00
The chemical equation below shows the reaction of sodium (na) and chlorine (cl) to form sodium chloride (nacl). 2na + cl2 → 2nacl in this equation, which of the following is a reactant? i. sodium ii. chlorine iii. sodium chloride
Answers: 1

You know the right answer?
The isomerization reaction CH3NC → CH3CN obeys the first-order rate law, rate = k[CH3NC], in the pre...
Questions


Physics, 12.03.2021 18:50

Spanish, 12.03.2021 18:50


Mathematics, 12.03.2021 18:50

Arts, 12.03.2021 18:50

Mathematics, 12.03.2021 18:50

Mathematics, 12.03.2021 18:50

Social Studies, 12.03.2021 18:50

Mathematics, 12.03.2021 18:50

Mathematics, 12.03.2021 18:50

English, 12.03.2021 18:50

Mathematics, 12.03.2021 18:50

Mathematics, 12.03.2021 18:50


Mathematics, 12.03.2021 18:50


Mathematics, 12.03.2021 18:50

Mathematics, 12.03.2021 18:50

English, 12.03.2021 18:50

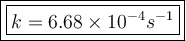
![\dfrac{d[CH_3NC]}{[CH_3NC]}=-kt](/tpl/images/0521/4081/74427.png)
![[CH_3NC]=[CH_3NC]_0e^{-kt}](/tpl/images/0521/4081/d933f.png)
![\dfrac{CH_3NC]}{[CH_3NC]_0}=0.72](/tpl/images/0521/4081/eda95.png)