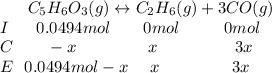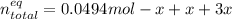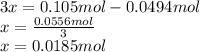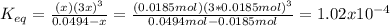Consider the decomposition of the compound C5H6O3 as follows: C5H6O3(g) C2H6(g) + 3CO(g) A 5.63 g sample of pure C5H6O3(g) was placed in an evacuated 2.50 L flask and heated to 200.ºC. At equilibrium, the pressure in the flask was 1.63 atm. Calculate K for this reaction.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:00
In a water molecule, hydrogen and oxygen are held together by a(an) bond. a) double covalent b) ionic c) nonpolar covalent d) hydrogen e) polar covalent
Answers: 1

Chemistry, 22.06.2019 14:10
16. in a reaction that has reached equilibrium, a. the forward and reverse reactions are occurring at the same rate. b. the reactants and products are in equal concentrations. c. the forward reaction has gone further than the reverse reaction. d. there are equal numbers of atoms on both sides of the equation. e. a, b, and d are correct.
Answers: 2

Chemistry, 22.06.2019 15:00
Which of the following is the correct formula for copper (i) sulfate trihydrate? cuso4 · 3h2o cuso4(h2o)3 cu2so4(h2o)3 cu2so4 · 3h2o
Answers: 1

You know the right answer?
Consider the decomposition of the compound C5H6O3 as follows: C5H6O3(g) C2H6(g) + 3CO(g) A 5.63 g...
Questions

Mathematics, 15.01.2021 08:30

Social Studies, 15.01.2021 08:30




Advanced Placement (AP), 15.01.2021 08:30


English, 15.01.2021 08:30


Medicine, 15.01.2021 08:30

Social Studies, 15.01.2021 08:30


Mathematics, 15.01.2021 08:30



Advanced Placement (AP), 15.01.2021 08:30


Chemistry, 15.01.2021 08:30


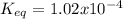
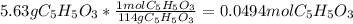
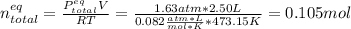
 ", which yields to:
", which yields to: