

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 14:30
Connect the whole numbers on the periodic table to indicate what they represent?
Answers: 3

Chemistry, 22.06.2019 22:30
The vapor pressure of ethanol is 1.00 × 102 mmhg at 34.90°c. what is its vapor pressure at 61.61°c? (δhvap for ethanol is 39.3 kj/mol.)
Answers: 2


Chemistry, 23.06.2019 05:30
Awhite powder is added to a solution. the images show observations made before the powder is added, just after the powder has been added, and a little while later. (the liquid in the small beaker is phenol red solution.) what evidence shows that a chemical change has taken place?
Answers: 1
You know the right answer?
Consider the following equilibrium for nitrous acid, HNO2, a weak acid: HNO2(aq) + H2O (l) —><...
Questions



Mathematics, 21.02.2020 18:32


English, 21.02.2020 18:32






Computers and Technology, 21.02.2020 18:32








Computers and Technology, 21.02.2020 18:33

Mathematics, 21.02.2020 18:33

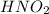 is weaker acid and have less tendency to dissociate in water.Hence, formation of reactants will be more favourable than the formation of products.When the acid is more diluted more number of hydronium ions are produced hence the direction of equilibrium will shift to forward direction.
is weaker acid and have less tendency to dissociate in water.Hence, formation of reactants will be more favourable than the formation of products.When the acid is more diluted more number of hydronium ions are produced hence the direction of equilibrium will shift to forward direction.

