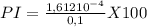Chemistry, 23.02.2020 05:56 isaacbryan2416
A certain weak acid, HA, has a Ka value of 2.6×10−7. Calculate the percent ionization of HA in a 0.10 M solution.

Answers: 3


Another question on Chemistry

Chemistry, 23.06.2019 01:50
Ablock of aluminum is dropped into a graduated cylinder with an initial volume of water at 75ml and the volumes rises to 90ml. if the block has a mass of 40.5 g what is its density ?
Answers: 1

Chemistry, 23.06.2019 04:00
If you are told to get 100 ml of stock solution to use to prepare smaller size sample for an experiment, which piece of glassware would you use?
Answers: 1

Chemistry, 23.06.2019 09:00
Avogradoa number was calculated by determining the number of atoms in?
Answers: 1

Chemistry, 23.06.2019 10:30
Can anyone explain 1. review your spectrometry data and use the known elements to identify the star's composition. which unknown elements make up this star? justify your element selections. 2. in parts i and ii of the lab, what happened to the electrons of each element to produce the different colors of light? explain your answers using important terms from the lesson and information provided in the laboratory. 3. stars composed of heavier (more massive) elements are often slightly older than stars made predominantly from hydrogen and helium. based on your data, is the newly discovered star a younger star? explain your answer.
Answers: 2
You know the right answer?
A certain weak acid, HA, has a Ka value of 2.6×10−7. Calculate the percent ionization of HA in a 0.1...
Questions

Biology, 07.10.2019 22:00


Geography, 07.10.2019 22:00

Business, 07.10.2019 22:00


Mathematics, 07.10.2019 22:00

Physics, 07.10.2019 22:00


Mathematics, 07.10.2019 22:00

Mathematics, 07.10.2019 22:00

Business, 07.10.2019 22:00



Mathematics, 07.10.2019 22:00

Chemistry, 07.10.2019 22:00

Social Studies, 07.10.2019 22:00



Mathematics, 07.10.2019 22:10

![PI=\frac{[A-]}{[HA]} x100](/tpl/images/0520/5455/031dd.png)
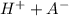
![Ka=\frac{[H+][A-]}{[HA]}\\ \\](/tpl/images/0520/5455/b886f.png)
![Ka=\frac{[H+]^{2} }{[HA]} \\\\](/tpl/images/0520/5455/c597e.png)
![[H+]=\sqrt[2]{Ka.[HA]} \\\\](/tpl/images/0520/5455/88b1b.png)
![[H+] =\sqrt{(2,610^{-7} )(0,1)} = 1,61210^{-4}](/tpl/images/0520/5455/d2b22.png)