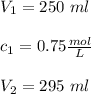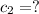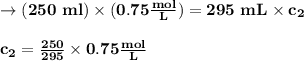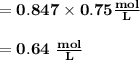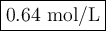Dilutions Worksheet - Solutions
If 45 mL of water are added to 250 mL of a 0.75 M K2SO4 soluti...

Chemistry, 23.02.2020 04:47 brainlord4209
Dilutions Worksheet - Solutions
If 45 mL of water are added to 250 mL of a 0.75 M K2SO4 solution, what will the
molarity of the diluted solution be?
(0.75 M)(250 ml) = M2 (295 mL)
M2 = (0.75 M) (250 mL) = 0.64 M
(295 mL)
Where did the 295ml came from

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
What is the most stable monatomic ion formed from nitrogen
Answers: 2

Chemistry, 22.06.2019 05:30
Transportation is the largest single source of air pollution in the united states. air pollution can harm the environment and human health. which technology could offer a solution to this problem? mufflers that reduce noise motors that run on electricity tires that improve gas mileage
Answers: 3

Chemistry, 22.06.2019 13:30
Some animals that try to adapt to climate changes eventually die due to starvation, as climate change alters the web.
Answers: 2

Chemistry, 22.06.2019 13:30
What does the xylem do? stores the glucose captures the sunlight absorbs oxygen into the leaf carries water from the roots to the leaves
Answers: 1
You know the right answer?
Questions

Mathematics, 12.08.2021 23:10





Geography, 12.08.2021 23:10

Mathematics, 12.08.2021 23:10

Mathematics, 12.08.2021 23:10

Mathematics, 12.08.2021 23:10

English, 12.08.2021 23:10

Social Studies, 12.08.2021 23:10

Mathematics, 12.08.2021 23:10



Mathematics, 12.08.2021 23:10





Mathematics, 12.08.2021 23:10