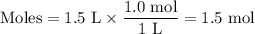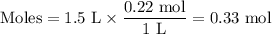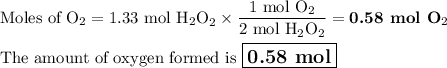Consider the following reaction:
2H2O2(aq)→2H2O(l)+O2(g)
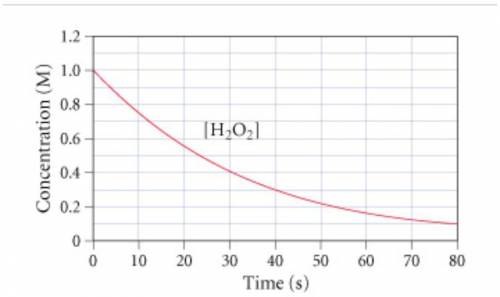
The graph (Figure 1) shows the...

Chemistry, 22.02.2020 19:58 MarishaTucker
Consider the following reaction:
2H2O2(aq)→2H2O(l)+O2(g)
The graph (Figure 1) shows the concentration of H2O2 as a function of time.
If the instantaneous rate of formation of O2 is 3.3*(10^-3) moles/(liters*seconds), then...
If the initial volume of the H2O2 solution is 1.5 L, what total amount of O2 (in moles) is formed in the first 50 s of reaction?
Express your answer using two significant figures.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
Which of the following pairs of elements belong to the same groupa. h and he b. li and bec. c and pb d. ga and ge
Answers: 1

Chemistry, 22.06.2019 07:30
All cells are made of four types of acids, lipids, proteins, and carbohydrates.
Answers: 1

Chemistry, 22.06.2019 14:10
Precision can be defined as the o exact center of a data set. o reproducibility of a measured value. o correlation between two variables that are measured in a data set agreement between a measured value and an accepted value.
Answers: 2

Chemistry, 23.06.2019 02:00
Now look at the segment of the graph between the two data points marked with black squares. describe how the boiling point and melting point plots behave between these points. be as specific as possible.
Answers: 1
You know the right answer?
Questions



Mathematics, 11.02.2020 02:59





Mathematics, 11.02.2020 02:59



Mathematics, 11.02.2020 02:59


English, 11.02.2020 02:59

Mathematics, 11.02.2020 02:59

Mathematics, 11.02.2020 02:59





Health, 11.02.2020 02:59