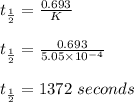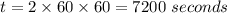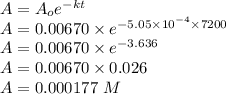Chemistry, 22.02.2020 04:59 francisebell2p698f2
Consider the first-order reaction described by the equation Cyclopropane gas isomerizes to propene gas. At a certain temperature, the rate constant for this reaction is 5.05 × 10 − 4 s − 1 . Calculate the half-life of cyclopropane at this temperature. t 1 / 2 = s Given an initial cyclopropane concentration of 0.00670 M , calculate the concentration of cyclopropane that remains after 2.00 hours. concentration.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:30
The characteristic of two different types of reactions are shown below. reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of and element. which statement is true about the atoms of the elements that participate in the two reactions? a: their identity changes in both reaction a and b. b: their identity changes in reaction a but not b. c: their identity changes in reaction b but not a. d: their identity remains the same.
Answers: 1

Chemistry, 22.06.2019 13:30
Ants live on acacia trees in south america. the ants feed on sugars secreted by the trees. the trees provide room for the ants to live. the ants sting any other insect or animal that comes to eat the trees. what type of relationship is this?
Answers: 1


Chemistry, 22.06.2019 18:00
Many pharmaceutical drugs are organic compounds that were originally synthesized in the laboratory. which two scientific disciplines are bridged by pharmaceutical drugs? chemistry and forensics chemistry and medicine biology and forensics biology and criminology
Answers: 2
You know the right answer?
Consider the first-order reaction described by the equation Cyclopropane gas isomerizes to propene g...
Questions






History, 04.09.2020 01:01


History, 04.09.2020 01:01



Mathematics, 04.09.2020 01:01


Social Studies, 04.09.2020 01:01







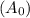 of was
of was 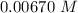 .
.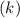 is
is 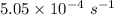
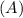 after 2 hours.
after 2 hours.