Chemistry, 22.02.2020 04:19 Mypasswordishotdog11
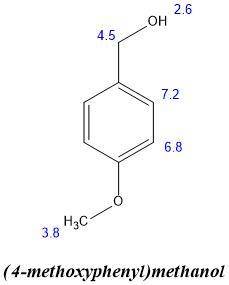
A compound, C8H10O2, has an 1H NMR spectrum showing peaks at delta 2.6 (1 H, singlet), 3.8 (3 H, singlet), 4.5 (2 H, singlet), 6.8 (2 H, doublet), and 7.2 (2 H, doublet). Draw its structure in the window below. a. You do not have to consider stereochemistry. b. You do not have to explicitly draw H atoms. c. Do not include lone pairs in your answer. They will not be considered in the grading. d. In cases where there is more than one answer, just draw one.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Drag each label to the correct location on the chart. classify each reaction as endothermic or exothermic.
Answers: 1

Chemistry, 22.06.2019 10:10
For the reaction, 4 a(g) + 3 b(g) => 2 c(g), the following data were obtained at constant temperature. experiment initial[a],mol/l initial [b],mol/l initial rate,m/min 1 0.200 0.150 5.00 2 0.400 0.150 10.0 3 0.200 0.300 10.0 4 0.400 0.300 20.0 which of the following is the correct rate law for the reaction? 1. rate = k[a]2[b]2 2. rate = k[a][b] 3. rate = k[a]2[b] 4. rate = k[a][b]2
Answers: 3


You know the right answer?
A compound, C8H10O2, has an 1H NMR spectrum showing peaks at delta 2.6 (1 H, singlet), 3.8 (3 H, sin...
Questions

Mathematics, 10.11.2020 20:00

Mathematics, 10.11.2020 20:00

Mathematics, 10.11.2020 20:00

Health, 10.11.2020 20:00

History, 10.11.2020 20:00

Geography, 10.11.2020 20:00

English, 10.11.2020 20:00

English, 10.11.2020 20:00



Mathematics, 10.11.2020 20:00



Computers and Technology, 10.11.2020 20:00


English, 10.11.2020 20:00


Mathematics, 10.11.2020 20:00