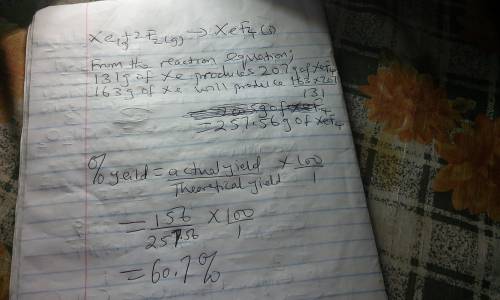Chemistry, 22.02.2020 01:45 cjtambasco
Although they were formerly called the inert gases, at least the heavier elements of Group 8 do form relatively stable compounds. For example, xenon combines directly with elemental fluorine at elevated temperatures in the presence of a nickel catalyst. Use table 1 and table 2. Xe(g) + 2 F2(g) → XeF4(s) What is the theoretical mass of xenon tetrafluoride that should form when 163 g of xenon is reacted with 164 g of F2? g= What is the percent yield if only 156 g of XeF4 is actually isolated?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1


Chemistry, 22.06.2019 21:30
What is happening when the water inside a kettle heats up and begins to boil
Answers: 1

Chemistry, 22.06.2019 21:50
Liquid from a brewery fermentation contains 10% ethanol and 90% water. part of the fermentation product (50,000 kg/h) is pumped to a distillation column on the factory site. under current operating conditions, a distillate of 45% ethanol and 55% water is produced from the top of the column at a rate of one-tenth that of the feed. what is the composition of the waste "bottoms" from the still?
Answers: 2
You know the right answer?
Although they were formerly called the inert gases, at least the heavier elements of Group 8 do form...
Questions


Biology, 02.02.2020 21:01

Physics, 02.02.2020 21:01


World Languages, 02.02.2020 21:01

History, 02.02.2020 21:01

History, 02.02.2020 21:01

Mathematics, 02.02.2020 21:01


Social Studies, 02.02.2020 21:01

Mathematics, 02.02.2020 21:01

Biology, 02.02.2020 21:01



Biology, 02.02.2020 21:01


History, 02.02.2020 21:01


Mathematics, 02.02.2020 21:01

History, 02.02.2020 21:01