Chemistry, 21.02.2020 21:57 usagimiller
The standard enthalpies of combustion of cis-2-hexene and trans-2-hexene (to form carbon dioxide and water) are −3727.9 kJ·mol−1 and −3726.3 kJ·mol−1, respectively. Calculate the enthalpy of the following isomerization process.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:00
Ican determine the molar mass of an element by looking on the under the atomic mass for the element. for example the molar mass of phosphorus is 30.974 grams/mole. avogadro’s number tells me the amount of representative particles in 1 mole of any substance. this means 12.011 gram sample of carbon and a 32.0 gram sample of sulfur have the same number of atoms.
Answers: 1

Chemistry, 22.06.2019 18:50
Which of the following is a conclusion that resulted from ernest rutherford’s scattering experiment? (will mark brainliest) a. the nucleus is negatively charged b. the atom is a dense solid and is indivisible c. the mass is conserved when atoms react chemically d. the nucleus is very small and the atom is mostly empty space
Answers: 3

Chemistry, 22.06.2019 19:30
Acetylene gas c2h2 undergoes combustion to produce carbon dioxide and water vapor how many grams of water are produced by the same amount of c2h2?
Answers: 2

You know the right answer?
The standard enthalpies of combustion of cis-2-hexene and trans-2-hexene (to form carbon dioxide and...
Questions


Mathematics, 12.10.2020 03:01



Biology, 12.10.2020 03:01


Mathematics, 12.10.2020 03:01


World Languages, 12.10.2020 03:01



French, 12.10.2020 03:01


Mathematics, 12.10.2020 03:01


Mathematics, 12.10.2020 03:01



Mathematics, 12.10.2020 03:01

Computers and Technology, 12.10.2020 03:01

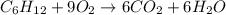
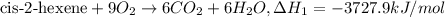 ..[1]
..[1]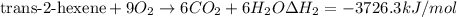 [2]
[2]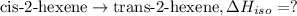 ...[3]
...[3]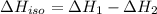 (Hess's law)
(Hess's law)