
Chemistry, 21.02.2020 21:34 kaoris2322
For each of the following cases, identify the order with respect to the reactant, A. Case (A > product)
1. The half-life of A increases as the initial concentration of A decreases.
2. A twofold increase in the initial concentration of A leads to a fourfold increase in the initial rate.
3.A twofold increase in the initial concentration of A leads to a 1.41-fold increase in the initial rate.
4.The time required for [A] to decrease from [A]0 to [A]0/2 is equal to the time required for [A] to decrease from [A]0/2 to [A]0/4.
5.The rate of decrease of [A] is constant.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 03:00
Flourine is found to undergo 10% radioactivity decay in 366 minutes determine its halflife
Answers: 3

Chemistry, 22.06.2019 17:50
You exhale co2 which is produced during cellular respiration. co2 combines with the water in your blood's plasma to make up one half of the body's most important buffer pair, carbonic acid. the more physical activity you engage in, the more co2 your body is producing. you can see this by putting some of the cabbage indicator in a glass and then blowing bubbles into it through a straw. can you see a change in the color of the indicator?
Answers: 2

Chemistry, 22.06.2019 19:30
How might this scientific phenomena be explained? a paper clip floats on water.
Answers: 1
You know the right answer?
For each of the following cases, identify the order with respect to the reactant, A. Case (A > pr...
Questions


Mathematics, 15.05.2020 08:57





Chemistry, 15.05.2020 08:57

Mathematics, 15.05.2020 08:57

Chemistry, 15.05.2020 08:57


Mathematics, 15.05.2020 08:57


Mathematics, 15.05.2020 08:57


Mathematics, 15.05.2020 08:57

Mathematics, 15.05.2020 08:57



Mathematics, 15.05.2020 08:57

English, 15.05.2020 08:57

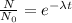
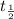 ∝
∝ ![\frac{1}{[A_{0} ]^{n-1} }](/tpl/images/0519/6244/24356.png)


