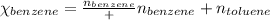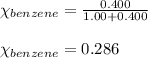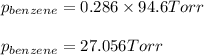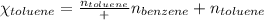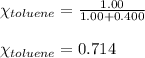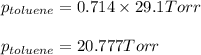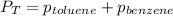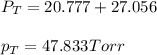Chemistry, 21.02.2020 17:21 labrandonanderson00
Benzene, C6H6, and toluene, C6H5CH3, form an ideal solution. The vapor pressure of benzene is 94.6 Torr and that of Topic 5C Exercises 372 Topic 5C Phase Equilibria in Two-Component Systems toluene is 29.1 Torr at 25 8C. What is the vapor pressure of each component at 25 8C and what is the total vapor pressure of a mix- ture of 1.00 mol benzene and 0.400 mol toluene at 25 8C?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:40
Which of the following pressures is equal to 760 mm hg? 2.0 atm 101.3 pa 101,300 kpa 101,300 pa
Answers: 2

Chemistry, 22.06.2019 03:30
Each pair of clay balls represents to planetesimals if each plane test molluscum pound of the same material and is separated by the same distance which pair experiences the greatest gravitational attraction
Answers: 2

Chemistry, 22.06.2019 17:00
In a heat engine of 1000 j of heat enters the system and the piston does 500 j of work what is the final internal energy of the system if the inital energy was 2000 j we have to do all of these down here 1)write the equation 2)list out your know variables 3)plug the numbers into the equations 4)solve 5)write your solution statemtn that includes inital energuy and final energuy added
Answers: 1

Chemistry, 23.06.2019 01:30
Will a solution form when the solvent and solute are both nonpolar? a. not likely b. never c. most likely
Answers: 1
You know the right answer?
Benzene, C6H6, and toluene, C6H5CH3, form an ideal solution. The vapor pressure of benzene is 94.6 T...
Questions

Chemistry, 20.10.2019 16:30



Health, 20.10.2019 16:30


Chemistry, 20.10.2019 16:30

Mathematics, 20.10.2019 16:30

Mathematics, 20.10.2019 16:30

Mathematics, 20.10.2019 16:30




Mathematics, 20.10.2019 16:30



Health, 20.10.2019 16:30

Mathematics, 20.10.2019 16:30

Social Studies, 20.10.2019 16:30

English, 20.10.2019 16:30

Mathematics, 20.10.2019 16:30

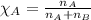
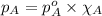 ........(1)
........(1)