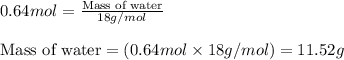Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 14:10
Aconcentrated solution of ammonia is 14.8m and has a density of 0.899g/l. what is the concentration of ammonia in this solution in weight percent (%w/w)?
Answers: 1


Chemistry, 22.06.2019 17:40
Which statement about hf is true? it is zero for any compound in its standard state. it is positive when the bonds of the product store more energy than those of the reactants. it is negative when a compound forms from elements in their standard states. it is zero for any element that is in the liquid state.
Answers: 1

Chemistry, 23.06.2019 10:00
The temperature of a lead fishing weight rises from 26 °c to 38 °c as it absorbs 11.3 j of heat. what is the mass of the fishing weight in grams?
Answers: 2
You know the right answer?
Trisilane (Si3H8) is a liquid with a density of 0.739 g cm-3. It reacts with oxygen to give silicon...
Questions



French, 09.11.2020 20:00

English, 09.11.2020 20:00

Mathematics, 09.11.2020 20:00




Biology, 09.11.2020 20:00

Geography, 09.11.2020 20:00


Business, 09.11.2020 20:00

English, 09.11.2020 20:00



Mathematics, 09.11.2020 20:00


Geography, 09.11.2020 20:00

SAT, 09.11.2020 20:00

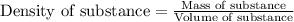
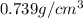
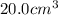
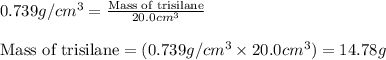
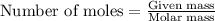 .....(1)
.....(1)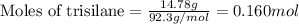
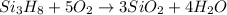
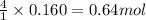 of water
of water