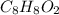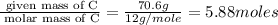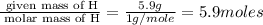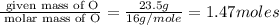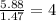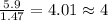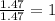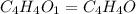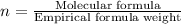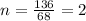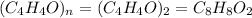Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:40
In the reading, yao chen-yuan describes traveling to deliver a message. why was he willing to risk danger to travelto tientsin? he wanted to the boxers with their cause
Answers: 2

Chemistry, 21.06.2019 18:20
Complete the table for ion charge based upon their losing or gaining electrons in the outer shell. (use the periodic table as necessary.) group most likely ionic charge # of valence electrons i +1 ii +2 iii +3 iv +4 or -4 v -3 vi -2 vii -1 viii 0
Answers: 2

Chemistry, 22.06.2019 06:00
Compare and contrast physical changes with chemical changes.
Answers: 1

You know the right answer?
A compound that is composed of carbon, hydrogen, and oxygen contains 70.6% C, 5.9% H, and 23.5% O by...
Questions

Computers and Technology, 11.02.2020 20:49

Biology, 11.02.2020 20:49

History, 11.02.2020 20:49



Mathematics, 11.02.2020 20:49




Mathematics, 11.02.2020 20:49


Health, 11.02.2020 20:49


Computers and Technology, 11.02.2020 20:49

Mathematics, 11.02.2020 20:49

Mathematics, 11.02.2020 20:49