Chemistry, 21.02.2020 05:58 skylarschumacher7
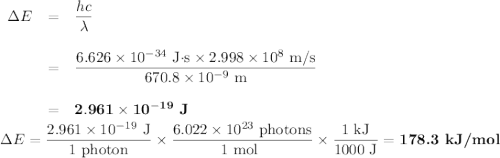
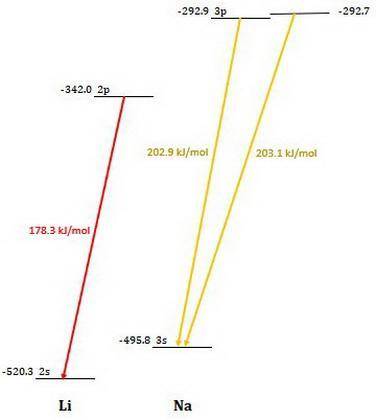
Calculate the photon energy (in kJ/mol) for the single Li emission and the two Na emission wavelengths. To accomplish this, first calculate the energy in units of /photon from Equation (4) and then multiply the result by Avogadro's number to express the energy in /mol of photons. Lastly, divide by 1000 to convert this result to units of kl/mol. Next, use the photon energies to determine the valence orbital energies for both Li and Na. For lithium, the transition is from the 2p- to the 2s-orbital, and the 2s-orbital energy is -520.3 kJ/mol. Use this to find the energy of the 2p-orbitals in Li. For sodium, the higher-energy photon is emitted when the electron drops from one of the 3p-orbitals to the 3s-orbital, while the lower energy photon is emitted when the electron drops from one of the 3d-orbitals to one of the 3p-orbitals. Use these facts, along with the known energy of the 3s-orbital (-495.8 kJ/mol), to find the energies of the 3p- and 3d-orbitals.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:40
In which environment would primary succession occur? a forest with a few remaining trees after a recent wildfire an area of exposed rock after a glacier melts away beach that is exposed to the air at low tide an abandoned baseball field in a small town
Answers: 1

Chemistry, 23.06.2019 13:30
How many more valence electrons does sodium need to have a full outer valence shell
Answers: 1

Chemistry, 23.06.2019 13:40
Match these items with their examples. 1. liquid solution milk 2. solid solution aluminum foil 3. compound soda 4. colloid steel 5. element salt
Answers: 1

You know the right answer?
Calculate the photon energy (in kJ/mol) for the single Li emission and the two Na emission wavelengt...
Questions

Mathematics, 14.09.2020 15:01

Mathematics, 14.09.2020 15:01

History, 14.09.2020 15:01

Mathematics, 14.09.2020 15:01

History, 14.09.2020 15:01

Mathematics, 14.09.2020 15:01

Mathematics, 14.09.2020 15:01

Social Studies, 14.09.2020 15:01

Social Studies, 14.09.2020 15:01

Mathematics, 14.09.2020 15:01

English, 14.09.2020 15:01

English, 14.09.2020 15:01

Biology, 14.09.2020 15:01

English, 14.09.2020 15:01

Mathematics, 14.09.2020 15:01

Computers and Technology, 14.09.2020 15:01

Mathematics, 14.09.2020 15:01

Mathematics, 14.09.2020 15:01

Social Studies, 14.09.2020 15:01

Mathematics, 14.09.2020 15:01