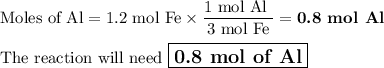Question 21
0.1 pts
Given the following equation:
2 Al(s) +3 FeO(s) 3Fe(s)+ Al2O3(...


Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Which describes fat? a: a carbohydrate that produces energy b: a nucleic acid that directs cell function c: a lipid that stores energy d: a protein that speeds up a chemical reaction
Answers: 1

Chemistry, 22.06.2019 10:50
How many liters of oxygen gas, at standard temperature and pressure, will react with 35.8 grams of iron metal? 4 fe (s) + 3 o₂ (g) → 2 fe₂o₃ (s)
Answers: 2

Chemistry, 22.06.2019 15:00
According to the diagram, what sources contribute to the phosphorus found in soil? according to the diagram, phosphorus found in soil contributes phosphorus to what other sources?
Answers: 1

Chemistry, 22.06.2019 19:30
Draw the lewis structure for the trisulfur s3 molecule. be sure to include all resonance structures that satisfy the octet rule.
Answers: 3
You know the right answer?
Questions

Geography, 11.09.2019 17:30


Mathematics, 11.09.2019 17:30


Mathematics, 11.09.2019 17:30


Biology, 11.09.2019 17:30

Mathematics, 11.09.2019 17:30


Mathematics, 11.09.2019 17:30

World Languages, 11.09.2019 17:30


Mathematics, 11.09.2019 17:30


English, 11.09.2019 17:30



History, 11.09.2019 17:30

Mathematics, 11.09.2019 17:30