
Chemistry, 21.02.2020 03:00 darenl4478
The Fe 2 + ( 55.845 g/mol) content of a 2.349 g steel sample dissolved in 50.00 mL of an acidic solution was determined by tiration with a standardized 0.100 M potassium permanganate ( KMnO 4 , 158.034 g/mol) solution. The titration required 43.16 mL to reach the end point. What is the concentration of iron in the steel sample? Express your answer as grams of Fe per gram of steel. MnO − 4 + 8 H + + 5 Fe 2 + − ⇀ ↽ − Mn 2 + + 5 Fe 3 + + 4 H 2 O

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:40
Astudent is given a sample of a blue copper sulfate hydrate. he weighs the sample in a dry covered porcelain crucible and got a mass of 23.875 g for the crucible, lid, and sample. the mass of the empty crucible and lid was found earlier to be 22.652 g. he then heats the crucible to expel the water of hydration, keeping the crucible at red heat for 10 minutes with the lid slightly ajar. on colling, he finds the mass of crucible, lid, and contents to be 23.403 g. the sample was changed in the process to very light clue anhydrous cuso4. if there are again 100.0 g of hydrate, how many grams of cuso4 are in it? how many moles of cuso4? (hint: molar mass of cuso4 = 159.6 g / mole. what per cent of the hydrate is cuso4? you may convert the mass of cuso4 to moles.)
Answers: 3

Chemistry, 22.06.2019 16:30
How many grams of mgbr2 are needed to produce 75g or metal?
Answers: 1


Chemistry, 23.06.2019 05:30
Awhite powder is added to a solution. the images show observations made before the powder is added, just after the powder has been added, and a little while later. (the liquid in the small beaker is phenol red solution.) what evidence shows that a chemical change has taken place?
Answers: 1
You know the right answer?
The Fe 2 + ( 55.845 g/mol) content of a 2.349 g steel sample dissolved in 50.00 mL of an acidic solu...
Questions

English, 13.04.2020 20:48

Mathematics, 13.04.2020 20:48

English, 13.04.2020 20:48

Law, 13.04.2020 20:49

English, 13.04.2020 20:49


History, 13.04.2020 20:49

History, 13.04.2020 20:49


Physics, 13.04.2020 20:49

Mathematics, 13.04.2020 20:50


History, 13.04.2020 20:50

Spanish, 13.04.2020 20:54




Mathematics, 13.04.2020 20:55


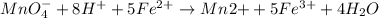
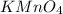 solution = 0.100 M
solution = 0.100 M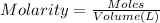
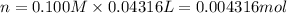
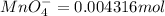
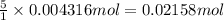 of ferrous ions
of ferrous ions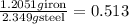 iron per gram of steel
iron per gram of steel

