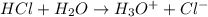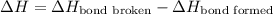Chemistry, 21.02.2020 02:31 chanelandme123
The O-H bond energy is 464 kJ/mol and the HI bond energy is 291 kJ/mol. Remembering that breaking any bond is an endothermic process and making any bond is exothermic, what is the net enthalpy change involved in the reaction between HI and H2O? Is this reaction endothermic or exothermic?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:20
Which of these statements explains the difference between nuclear binding energy and the strong nuclear force ?
Answers: 3

Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1


Chemistry, 23.06.2019 03:00
Determine type of reaction & predict the product c3h12+o2 =
Answers: 1
You know the right answer?
The O-H bond energy is 464 kJ/mol and the HI bond energy is 291 kJ/mol. Remembering that breaking an...
Questions



English, 31.01.2020 03:01

Mathematics, 31.01.2020 03:01

Advanced Placement (AP), 31.01.2020 03:01





Biology, 31.01.2020 03:01



Health, 31.01.2020 03:01


Biology, 31.01.2020 03:01

English, 31.01.2020 03:01


Mathematics, 31.01.2020 03:01


Mathematics, 31.01.2020 03:01