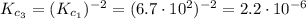Chemistry, 21.02.2020 02:21 bluefish743
Be sure to answer all parts. Enter your answers in scientific notation. At a particular temperature, Kc = 6.7 × 102 for 2NO(g) + 2H2(g) ⇌ N2(g) + 2H2O(g) Calculate Kc for each of the following reactions:(a) NO(g) + H2(g)1/2 N2(g) + H2O(g)
(b) 2 N2(g) + 4 H2O(g)4 NO(g) + 4 H2(g)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:30
98 ! and brainliest plz ! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 22.06.2019 20:00
Phosphoric acid is a triprotic acid ( =6.9×10−3 , =6.2×10−8 , and =4.8×10−13 ). to find the ph of a buffer composed of h2po−4(aq) and hpo2−4(aq) , which p value should be used in the henderson–hasselbalch equation? p k a1 = 2.16 p k a2 = 7.21 p k a3 = 12.32 calculate the ph of a buffer solution obtained by dissolving 18.0 18.0 g of kh2po4(s) kh 2 po 4 ( s ) and 33.0 33.0 g of na2hpo4(s) na 2 hpo 4 ( s ) in water and then diluting to 1.00 l.
Answers: 3

Chemistry, 22.06.2019 22:30
Which process describes vaporization that takes place below the surface of a liquid? condensation melting boiling evaporation
Answers: 1

You know the right answer?
Be sure to answer all parts. Enter your answers in scientific notation. At a particular temperature,...
Questions

Mathematics, 06.05.2021 22:30

Chemistry, 06.05.2021 22:30

Mathematics, 06.05.2021 22:30

History, 06.05.2021 22:30


Mathematics, 06.05.2021 22:30


Mathematics, 06.05.2021 22:30

Mathematics, 06.05.2021 22:30



English, 06.05.2021 22:30


Mathematics, 06.05.2021 22:30



Chemistry, 06.05.2021 22:30


Mathematics, 06.05.2021 22:30

Chemistry, 06.05.2021 22:30

![K_{c_{1}} = \frac{[N_{2}][H_{2}O]^{2}}{[NO]^{2}[H_{2}]^{2}} = 6.7 \cdot 10^{2}](/tpl/images/0518/6004/5a191.png) (2)
(2) ![K_{c_{2}} = \frac{[N_{2}]^{1/2}[H_{2}O]}{[NO][H_{2}]}](/tpl/images/0518/6004/75dc6.png) (4)
(4) ![\frac{[N_{2}]^{1/2}[H_{2}O]}{[NO][H_{2}]} = (\frac{[N_{2}][H_{2}O]^{2}}{[NO]^{2}[H_{2}]^{2}})^{1/2}](/tpl/images/0518/6004/6139f.png)
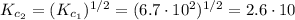
![K_{c_{3}} = \frac{[NO]^{4}[H_{2}]^{4}}{[N_{2}]^{2}[H_{2}O]^{4}}](/tpl/images/0518/6004/de89d.png) (6)
(6) ![\frac{[NO]^{4}[H_{2}]^{4}}{[N_{2}]^{2}[H_{2}O]^{4}} = \left(\frac{[N_{2}][H_{2}O]^{2}}{[NO]^{2}[H_{2}]^{2}}\right)^{-1}](/tpl/images/0518/6004/bc59e.png)
![\frac{[NO]^{4}[H_{2}]^{4}}{[N_{2}]^{2}[H_{2}O]^{4}} = \left(\frac{[NO]^{2}[H_{2}]^{2}}{[N_{2}][H_{2}O]^{2}}\right)^{2}](/tpl/images/0518/6004/80e13.png)