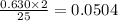Chemistry, 21.02.2020 01:24 INEEDTOPASSS
Determine the balanced chemical equation for this reaction. C8H18(g)+O2(g)→CO2(g)+H2O(g)
Part A:
Enter the coefficients for each compound in order, separated by commas. For example, 1,2,3,4 would indicate one mole of C8H18, two moles of O2, three moles of CO2, and four moles of H2O.
2,25,16,18
Part B:
0.280 mol of octane is allowed to react with 0.630 mol of oxygen. Which is the limiting reactant?
Oxygen
Part C:
How many moles of water are produced in this reaction?
H2O produced = 0.454 mol
Part D:
After the reaction, how much octane is left?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 19:30
Describe the forces both attractive and repulsive that occur as two atoms move closer together.
Answers: 1

Chemistry, 22.06.2019 21:30
The solid xy decomposes into gaseous x and y: xy(s) m x(g) + y(g) kp = 4.1 (at 0 °c) if the reaction is carried out in a 22.4 l container, which initial amounts of x and y will result in the formation of solid xy?
Answers: 1

Chemistry, 23.06.2019 00:30
Element j is 1s 2s 2p 3s . (i) how many unpaired electrons does j have? (ii) is j a good oxidizing agent or a reducing agent? (iii) state reason for the answer.
Answers: 1

Chemistry, 23.06.2019 06:30
Which of the following is true about the products formed during photosynthesis? (5 points) select one: a. they have the same mass as the mass of reactants. b. they are the same set of compounds as the reactants. c. they have more mass than the mass of reactants. d. they are chemically the same as the reactants.
Answers: 1
You know the right answer?
Determine the balanced chemical equation for this reaction. C8H18(g)+O2(g)→CO2(g)+H2O(g)
...
...
Questions

Mathematics, 12.10.2019 09:30


Physics, 12.10.2019 09:30


Mathematics, 12.10.2019 09:30

English, 12.10.2019 09:30


Mathematics, 12.10.2019 09:30


Mathematics, 12.10.2019 09:30



Mathematics, 12.10.2019 09:30


Mathematics, 12.10.2019 09:30

Mathematics, 12.10.2019 09:30


Computers and Technology, 12.10.2019 09:30


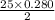 moles of oxygen
moles of oxygen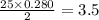
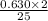 moles of octane will react with the 0.630 mole of oxygen present
moles of octane will react with the 0.630 mole of oxygen present