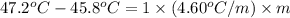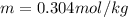Chemistry, 21.02.2020 00:31 athenajames1221
G A student enters the lab and determines the freezing point of pure liquid to be 47.2 ºC. A nonelectrolyte unknown substance is added to the liquid, and the freezing point of the solution is determined to be 45.8 ºC. If the freezing point depression constant for the solvent is 4.60 ºC/molal, what is the molality of the solution?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Write the symbol for every chemical element that has atomic number greater than 3 and atomic mass less than 12.0 u.
Answers: 1

Chemistry, 22.06.2019 09:50
Achemist has dissolved a certain substance in water. the chemist knows that more of the substance could be dissolved into the water before it stops dissolving. therefore
Answers: 2


Chemistry, 23.06.2019 05:00
He nucleus contains the cells genetic material in the form of dna. dna is organized into our chromosomes, which are made up of thousands of that determine our traits.
Answers: 1
You know the right answer?
G A student enters the lab and determines the freezing point of pure liquid to be 47.2 ºC. A nonelec...
Questions

Mathematics, 30.06.2021 21:30


Mathematics, 30.06.2021 21:30

Social Studies, 30.06.2021 21:30

Mathematics, 30.06.2021 21:30

Mathematics, 30.06.2021 21:30

Mathematics, 30.06.2021 21:30

Health, 30.06.2021 21:30


Mathematics, 30.06.2021 21:30

Mathematics, 30.06.2021 21:30

Law, 30.06.2021 21:30


Chemistry, 30.06.2021 21:30

Mathematics, 30.06.2021 21:30



Advanced Placement (AP), 30.06.2021 21:30


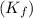 for solvent =
for solvent = 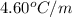
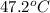
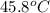
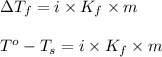
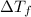 = change in freezing point
= change in freezing point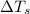 = freezing point of solution =
= freezing point of solution = 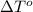 = freezing point of pure liquid =
= freezing point of pure liquid = 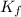 = freezing point constant for solvent =
= freezing point constant for solvent =