
Please use the values in the resources listed below instead of the textbook values. Under certain conditions the decomposition of ammonia on a metal surface gives the following data.[NH3] (M) 2.0 ✕ 10−3 4.0 ✕ 10−3 6.0 ✕ 10−3 Rate (mol/L/h) 1.5 ✕ 10−6 1.5 ✕ 10−6 1.5 ✕ 10−6 Determine the rate equation for this reaction. (Rate expressions take the general form: rate = k . [A]a . [B]b.)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Consider the point on the plot where 10.0 g of naoh have been added. what amount of naoh, in moles, has been added? 0.308 mol fecl3 initially present
Answers: 1

Chemistry, 22.06.2019 09:50
Although respiratory organs vary across different organisms, they all contain respiratory surfaces that have a large surface area and are extremely thin. explain why having an extremely thin respiratory surface with a large surface area is advantageous for the process of gas exchange
Answers: 1

Chemistry, 22.06.2019 12:00
In a laboratory, 1.55mg of an organic compound containing carbon, hydrogen, and oxygen is burned for analysis. this combustion resulted in the formation of 1.45mg of carbon dioxide and .89 mg of water. what is the empirical formula for this compound?
Answers: 1

Chemistry, 22.06.2019 16:10
Given the following equation: 2a1 + 3mgcl2 --> 2alcl3 + 3mg how many moles of aluminum chloride are produced from 2.5 moles of magnesium chloride?
Answers: 1
You know the right answer?
Please use the values in the resources listed below instead of the textbook values. Under certain co...
Questions


Physics, 23.09.2019 07:30


Biology, 23.09.2019 07:30


Computers and Technology, 23.09.2019 07:30




Mathematics, 23.09.2019 07:30


Physics, 23.09.2019 07:30

Biology, 23.09.2019 07:30


Health, 23.09.2019 07:30

Mathematics, 23.09.2019 07:30

English, 23.09.2019 07:30

Health, 23.09.2019 07:30


![R=k[NH_3]^0](/tpl/images/0518/3680/fda84.png)
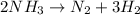
![R=k[NH_3]^x](/tpl/images/0518/3680/194a4.png)
![[NH_3]=2.0\times 10^{-3} M](/tpl/images/0518/3680/fed44.png)
![1.5\times 10^{-6} M/s=k[2.0\times 10^{-3} M]^x](/tpl/images/0518/3680/2ac63.png) ..[1]
..[1]![[NH_3]=4.0\times 10^{-3} M](/tpl/images/0518/3680/8a2bc.png)
![1.5\times 10^{-6} M/s=k[4.0\times 10^{-3} M]^x](/tpl/images/0518/3680/49a41.png) ..[2]
..[2]![\frac{1.5\times 10^{-6}M/s}{1.5\times 10^{-6}M/s}=\frac{k[2.0\times 10^{-3}M]^x}{k[4.0\times 10^{-3}M]^x}](/tpl/images/0518/3680/c6074.png)


