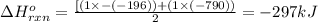Chemistry, 20.02.2020 23:42 xxleeciexx
Use the ΔHrxn values of the following reactions: 2SO2(g) + O2(g) → 2SO3(g) ΔHrxn = –196 kJ 2S(s) + 3O2(g) → 2SO3(g) ΔHrxn = –790 kJ to calculate the ΔHrxn value of this reaction: S(s) + O2(g) → SO2(g) ΔHrxn = ?

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 20:30
From the choices provided below, list the reagent(s) in order that will react with cyclopentanone to form the compound shown below.
Answers: 2

Chemistry, 23.06.2019 00:00
What conclusion can you draw from this experiment about the components of the black ink?
Answers: 3

Chemistry, 23.06.2019 07:00
Achemist who studies water samples did a demonstration of how to test for lead in water. she added a clear solution of potassium iodide to a clear solution of lead nitrate. then a yellow swirling solid formed in the liquid. what is most likely true about the yellow solid?
Answers: 3
You know the right answer?
Use the ΔHrxn values of the following reactions: 2SO2(g) + O2(g) → 2SO3(g) ΔHrxn = –196 kJ 2S(s) + 3...
Questions



Mathematics, 22.08.2019 02:10




Biology, 22.08.2019 02:10


Mathematics, 22.08.2019 02:10


Law, 22.08.2019 02:10

Mathematics, 22.08.2019 02:10

English, 22.08.2019 02:10



Law, 22.08.2019 02:10

Mathematics, 22.08.2019 02:10

Law, 22.08.2019 02:10

Law, 22.08.2019 02:10

Law, 22.08.2019 02:10

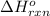 for the reaction is -297 kJ.
for the reaction is -297 kJ.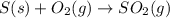
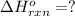
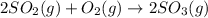
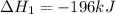
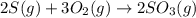
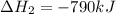
![\Delta H^o_{rxn}=\frac{[1\times (-\Delta H_1)]+[1\times \Delta H_2]}{2}](/tpl/images/0518/2732/98145.png)