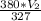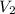OFFERING 50 POINTS
If a balloon containing 1,200 L of gas at 25°C and 760 mmHg pressure ascend...

Chemistry, 20.02.2020 23:26 emmaschloegl21x
OFFERING 50 POINTS
If a balloon containing 1,200 L of gas at 25°C and 760 mmHg pressure ascends to an altitude where the pressure is 380 mmHg and the temperature is 54°C, the volume will be: (Be sure to use the correct number of significant figures.) 3.8 x 10 -4 L 2,200 L 660 L 2,600 L

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Select the correct answer. when carbon dioxide dissolves in water, it sometimes reacts with water to form carbonic acid as in this balanced equation: co2 + h2o → h2co3. if 495 milliliters of carbon dioxide at 25°c and 101.3 kilopascals reacts with excess water, what is the theoretical yield of carbonic acid? use the periodic table and the ideal gas resource a. 0.889 g b. 1.10g c. 1.27g d. 2.02g what's the answer! quick!
Answers: 1

Chemistry, 22.06.2019 10:00
Miner's coal distributors does not mine coal itself, nor does it even store or handle the coal. instead, miner's solicits orders for low sulfur coal from other firms, then purchases the required amount from suppliers and directs them to ship the coal to its customers. what is miner's
Answers: 1

Chemistry, 22.06.2019 15:00
How is the shape of the poem “peer” connected to its meaning?
Answers: 2

You know the right answer?
Questions



Mathematics, 29.07.2021 02:00

Arts, 29.07.2021 02:00



Mathematics, 29.07.2021 02:00






Mathematics, 29.07.2021 02:00

Computers and Technology, 29.07.2021 02:00

Mathematics, 29.07.2021 02:00


Mathematics, 29.07.2021 02:00


Mathematics, 29.07.2021 02:00

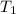 ) = 25°C = (25 + 273) K = 298K
) = 25°C = (25 + 273) K = 298K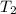 ) = 54°C = (54 + 273) K = 327 K
) = 54°C = (54 + 273) K = 327 K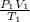 =
= 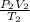
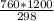 =
=