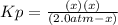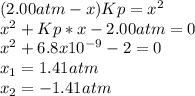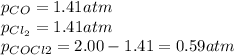Chemistry, 20.02.2020 23:05 transfergiecek8765
Lexan is a plastic used to make compact discs, eyeglass lenses, and bullet-proof glass. One of the compounds used to make Lexan is phosgene (COCl2), an extremely poisonous gas. Phosgene decomposes by the following reaction for which Kp = 6.8 ✕ 10-9 at 100°C. COCl2(g) equilibrium reaction arrow CO(g) + Cl2(g) If pure phosgene at an initial pressure of 2.0 atm decomposes, calculate the equilibrium pressures of all species.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:00
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change
Answers: 3

Chemistry, 22.06.2019 08:00
Me i dont know what to do! the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1

Chemistry, 22.06.2019 11:30
Determine the reaction and balance the following equations urgent due in the morning
Answers: 2

Chemistry, 22.06.2019 13:30
1) which of the following is the best example of a physical change? a) sugar dissolving in tea b) firefly glowing 2) in the combustion of ethane, what is/are the reactants? c2h6 + o2 ==> co2 + h2o a) c2h6 and o2 b) co2 and c2h6
Answers: 2
You know the right answer?
Lexan is a plastic used to make compact discs, eyeglass lenses, and bullet-proof glass. One of the c...
Questions

Mathematics, 06.04.2021 08:00


English, 06.04.2021 08:00






History, 06.04.2021 08:00

Computers and Technology, 06.04.2021 08:00

Mathematics, 06.04.2021 08:00

Mathematics, 06.04.2021 08:00

Mathematics, 06.04.2021 08:00


Mathematics, 06.04.2021 08:00

Mathematics, 06.04.2021 08:00

Chemistry, 06.04.2021 08:00

Chemistry, 06.04.2021 08:00

Biology, 06.04.2021 08:00

Mathematics, 06.04.2021 08:00

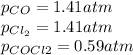
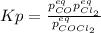
 , the law of mass action becomes:
, the law of mass action becomes: