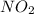Chemistry, 20.02.2020 20:58 ekarlewicz3343
Nitrogen and oxygen form an extensive series of oxides with the general formula NxOy. What is the empirical formula for an oxide that contains 30.44% by mass nitrogen?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 18:00
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 3

Chemistry, 22.06.2019 22:10
Determine the ph of 0.10 m nh3 solution. nh3 is a weak base with a kb equal to 1.8 x 10-5 round answer to nearest whole number.
Answers: 1


Chemistry, 23.06.2019 00:30
Fred is studying a substance that is made out of only one element. this means that
Answers: 1
You know the right answer?
Nitrogen and oxygen form an extensive series of oxides with the general formula NxOy. What is the em...
Questions








Computers and Technology, 27.11.2019 23:31











History, 27.11.2019 23:31