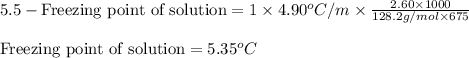Chemistry, 20.02.2020 20:35 ICAMARON632
The freezing point of benzene is 5.5°C. What is the freezing point of a solution of 2.60 g of naphthalene (C10H8) in 675 g of benzene (Kf of benzene = 4.90°C/m)?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Acamcorder has a power rating of 17 watts. if the output voltage from its battery is 7 volts, what current does it use?units:
Answers: 1

Chemistry, 22.06.2019 09:00
Given the following reaction: c3h8+5o2=3co2+4h20 how many grams of co2 will be produced 7 g of c3h8 and 98 g of o2
Answers: 1

Chemistry, 22.06.2019 16:50
Which of the following is an indication that a substance has undergone a chemical change? a. no new product has been formed. b. the color of the substance has not changed. c. the original constitute has not changed. d. the molecular structure has changed.
Answers: 1

Chemistry, 22.06.2019 21:20
40dm3 of gas at 760 torr are heated from 5°c to 50°c what is the new volume
Answers: 3
You know the right answer?
The freezing point of benzene is 5.5°C. What is the freezing point of a solution of 2.60 g of naphth...
Questions

English, 26.08.2019 01:30

Mathematics, 26.08.2019 01:30

Mathematics, 26.08.2019 01:30

Mathematics, 26.08.2019 01:30

Mathematics, 26.08.2019 01:30

French, 26.08.2019 01:30

English, 26.08.2019 01:30

English, 26.08.2019 01:30





Mathematics, 26.08.2019 01:30

Business, 26.08.2019 01:30


Advanced Placement (AP), 26.08.2019 01:30



Mathematics, 26.08.2019 01:30

Mathematics, 26.08.2019 01:30

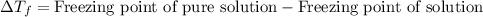
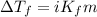
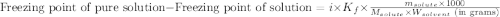
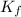 = molal freezing point elevation constant = 4.90°C/m
= molal freezing point elevation constant = 4.90°C/m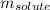 = Given mass of solute (naphthalene) = 2.60 g
= Given mass of solute (naphthalene) = 2.60 g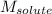 = Molar mass of solute (naphthalene) = 128.2 g/mol
= Molar mass of solute (naphthalene) = 128.2 g/mol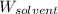 = Mass of solvent (benzene) = 675 g
= Mass of solvent (benzene) = 675 g