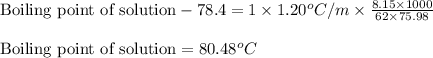Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Which statements are true about electrolysis? check all that apply. electrolysis requires an acid be present. electrolysis is described by two half-reactions. electrolysis is not an industrial process. electrolysis results in commercially valuable products. electrolysis involves the transfer of electrons. reduction results in the loss of electrons. oxidation results in the loss of electrons.
Answers: 1

Chemistry, 21.06.2019 22:30
Determine the wavelength of the light absorbed when an electron in a hydrogen atom makes a transition from an orbital in the n=3 level to an orbital in the n=7 level.
Answers: 2

Chemistry, 22.06.2019 08:00
What is the molarity of 60.0 grams of naoh dissolved in 750 milliliters of water? a) 1.1 m b) 2.0 m c) 12 m d) 75 m
Answers: 1

Chemistry, 22.06.2019 08:30
Which of the following would have less momentum than a 52 kg cheetah running at 10 m/s?
Answers: 2
You know the right answer?
Calculate the freezing point and boiling point of a solution containing 8.15 g of ethylene glycol (C...
Questions

Biology, 14.08.2020 21:01

Mathematics, 14.08.2020 21:01


Mathematics, 14.08.2020 21:01



Mathematics, 14.08.2020 21:01

Chemistry, 14.08.2020 21:01




Mathematics, 14.08.2020 21:01


Mathematics, 14.08.2020 21:01

History, 14.08.2020 21:01


Mathematics, 14.08.2020 21:01



Mathematics, 14.08.2020 21:01

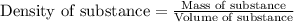
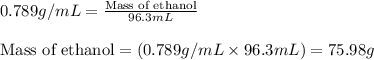
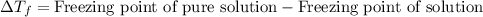
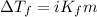
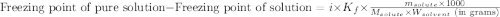
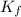 = molal freezing point elevation constant = 1.99°C/m
= molal freezing point elevation constant = 1.99°C/m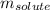 = Given mass of solute (ethylene glycol) = 8.15 g
= Given mass of solute (ethylene glycol) = 8.15 g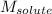 = Molar mass of solute (ethylene glycol) = 62 g/mol
= Molar mass of solute (ethylene glycol) = 62 g/mol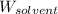 = Mass of solvent (ethanol) = 75.98 g
= Mass of solvent (ethanol) = 75.98 g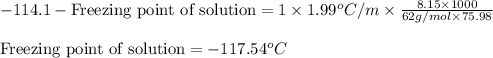
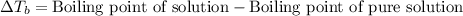
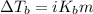
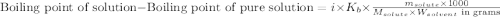
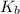 = molal boiling point elevation constant = 1.20°C/m.g
= molal boiling point elevation constant = 1.20°C/m.g