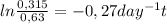Chemistry, 20.02.2020 16:50 meandmycousinmagic
A city's water supply is contaminated with a toxin at a concentration of 0.63 mg/L. Fortunately, this toxin decomposes to a safe mixture of products by first-order kinetics with a rate constant of 0.27 day–1. How long will it take for half of the toxin to decompose?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Does the temperature affect the solubility of sugar and salt in water? if it does tell me like different temperatures with different solubilities so i can sketch down a graph
Answers: 2


Chemistry, 22.06.2019 09:00
George is a dalmatian puppy. describe what happens to light that allows you to see george’s black and white coat.
Answers: 1

Chemistry, 23.06.2019 05:00
Question 5 match each term to its description. match term definition excess reactant a) reactant that can produce a lesser amount of the product limiting reactant b) reactant that can produce more of the product theoretical yield c) amount of product predicted to be produced by the given reactants
Answers: 2
You know the right answer?
A city's water supply is contaminated with a toxin at a concentration of 0.63 mg/L. Fortunately, thi...
Questions

Mathematics, 15.07.2019 20:10





Computers and Technology, 15.07.2019 20:10





Computers and Technology, 15.07.2019 20:10





Mathematics, 15.07.2019 20:10




![ln\frac{[A]_t}{[A]_0} = -kt](/tpl/images/0517/4629/57ff6.png)