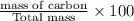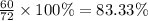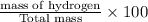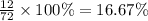Chemistry, 20.02.2020 08:29 ARAYAMYHAND
A hydrocarbon is a compound that contains mostly carbon and hydrogen. Calculate the percent composition (by mass) of the following hydrocarbon: C5H12. Enter the percentages of carbon and hydrogen numerically to four significant figures, separated by commas. View Available Hint(s)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Type the correct answer in the box. spell all words correctly.what is the correct term for living the most sustainable life you can within your current circumstances? when your are being as sustainable as you can within your current lifestyle, you are said to be sustainability.
Answers: 3

Chemistry, 22.06.2019 23:10
Afusion reaction takes place between carbon and another element. neutrons are released, and a different element is formed. the different element is a) lighter than helium.b)heavier than helium.c)the same weight as helium.d)dependent on the element that reacted with carbon.
Answers: 3


Chemistry, 23.06.2019 01:00
Which polymers are most closely related? a. protein and nucleic acids b. cellulose and starch c. nucleic acids and starch d. nucleic acids and cellulose
Answers: 2
You know the right answer?
A hydrocarbon is a compound that contains mostly carbon and hydrogen. Calculate the percent composit...
Questions



Mathematics, 21.06.2019 15:00









Mathematics, 21.06.2019 15:00

Mathematics, 21.06.2019 15:00







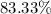 and
and 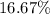 respectively.
respectively.