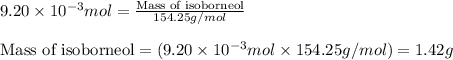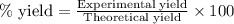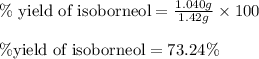Chemistry, 20.02.2020 08:02 tahjaybenloss16
Consider the sodium borohydride reduction of camphor to isoborneol. A reaction was performed in which 1.400 1.400 g of camphor was reduced by an excess of sodium borohydride to make 1.040 1.040 g of isoborneol. Calculate the theoretical yield and percent yield for this reaction.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:30
Mike and mitchell decide to have a foot race. they mark off a stretch of 100 yards, and recruit cindy to work the stopwatch. after running the race and looking at the results, cindy declared that mitchell was the fastest. so how did the boys times compare?
Answers: 3

Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1

Chemistry, 22.06.2019 15:30
Light waves can move through , but they travel fastest when they move through a(n) .
Answers: 1

Chemistry, 23.06.2019 05:00
What is dhmo? hint: you find it everywhere something is wet..
Answers: 1
You know the right answer?
Consider the sodium borohydride reduction of camphor to isoborneol. A reaction was performed in whic...
Questions








Mathematics, 23.09.2020 14:01

Mathematics, 23.09.2020 14:01

History, 23.09.2020 14:01

Mathematics, 23.09.2020 14:01


Health, 23.09.2020 14:01



Arts, 23.09.2020 14:01




Mathematics, 23.09.2020 14:01

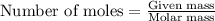 .....(1)
.....(1)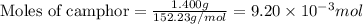
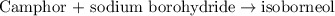
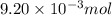 of camphor will produce =
of camphor will produce = 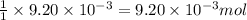 of isoborneol
of isoborneol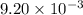 moles
moles