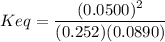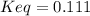Chemistry, 20.02.2020 06:15 aliyahmuhammad5197
An aqueous mixture of phenol and ammonia has initial concentrations of 0.302 M C6H5OH(aq) and 0.139 M NH3(aq). At equilibrium, the C6H5O–(aq) concentration is 5.00E-2 M. Calculate K for the reaction.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 23:00
What element has similar physical and chemical properties as boron.
Answers: 1

Chemistry, 23.06.2019 03:00
You have a sample of a metal, the sample is exactly 6.02 x 1023atom, if the sample has a mass 55.85 what metal is your sample made of?
Answers: 2

Chemistry, 23.06.2019 04:00
Achemical reaction is done in the setup shown , resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 2

Chemistry, 23.06.2019 04:31
Which molecules are more strongly attracted to one another -c3h8o molecules that make up liquid rubbing alcohol or ch4 molecules that make up methane gas
Answers: 3
You know the right answer?
An aqueous mixture of phenol and ammonia has initial concentrations of 0.302 M C6H5OH(aq) and 0.139...
Questions


Mathematics, 30.04.2021 18:30

Chemistry, 30.04.2021 18:30



Physics, 30.04.2021 18:30







Biology, 30.04.2021 18:30



Mathematics, 30.04.2021 18:30


English, 30.04.2021 18:30

Chemistry, 30.04.2021 18:30

Mathematics, 30.04.2021 18:30

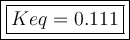
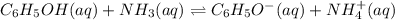
![Keq=\dfrac{[C_6H_5OH(aq)].[NH_{3}(aq)]}{[C_6H_5O^-(aq)].[NH_4^+(aq)]}](/tpl/images/0517/0091/ca7b5.png)