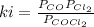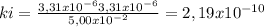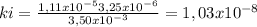Chemistry, 20.02.2020 05:59 kayranicole1
At 100oC the equilibrium constant for the reaction below has the value of Keq = 2.19 x 10−10. COCl2(g) ⇄ CO(g) + Cl2(g) Are the following mixtures of COCl2, CO, and Cl2 at equilibrium? If not, indicate the direction that the reaction must proceed to achieve equilibrium. (i) PCOCl2 = 5.00 × 10−2 atm; PCO = 3.31 × 10−6 atm; PCl2 = 3.31 × 10−6 atm (ii) PCOCl2 = 3.50 × 10−3 atm; PCO = 1.11 × 10−5 atm; PCl2= 3.25 × 10−6 atm
(i) not at equilibrium, left to right (ii) equilibrium
(i) equilibrium, (ii) not at equilibrium, right to left
(i) equilibrium, (ii) not at equilibrium, left to right
(i) not at equilibrium, right to left, (ii) equilibrium
(i) equilibrium, (ii) equilibrium

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 1

Chemistry, 22.06.2019 06:30
Ineed someone to see if my answers are correct! if any are wrong let me know what the correct answers would be and how to get that answer! 1. how many moles of sodium chloride are in 28 grams od nacl? a. 265 mole naclb. 856 mole naclc. 479 mole of nacld. 1.2 mole nacl < my choice2. 734 grams of lithium sulfate (li2so4) are dissolved to make 2500 ml of solution what is rhe molaratiy? a. 2.67 mb. 4.56 mc. 3.89 m < my choiced. 1.78 m3. how many grams of cacl2 would be dissolved in 3.0 l of a 0.50 m solution of cacl2? a. 250 g cacl2 b. 166.5 g cacl2c. 113.65 g cacl2d. 98 g cacl2 < my choice4. suppose you had 58.44 g of nacl and you dissolved it in exactly 2.00 liters. the molarity if the solution would be 0.5 mtrue < my choicefalse 5. i would need 22g of naoh to make a 3.0 m solution using 250 ml of solvent.true < my choicefalse6. identify the solute: you have a .0195 m solution made from using 6.5 g of solute and 3 l of solvent. identify the solute by solving for molar weight.a. the solute is nacl because the molar weight is 58.43 g/mol < my choiceb. the solute is h2so4 because the molar weight is 98.06 g/molc. the solute is cacl2 because the molar weight is 111.11 g/mol
Answers: 1

Chemistry, 22.06.2019 15:30
Two metal blocks that have slightly different temperatures are placed next to one another. after five minutes, they both have lower but equal temperatures. according to the law of conservation of energy, what most likelyhappened? energy was created inside the blocks.energy was destroyed inside the blocks.energy was absorbed into the blocks from outside the system.energy was transferred from the warmer block to the cooler block.
Answers: 2

Chemistry, 22.06.2019 15:30
Which statement names the physical property of wood a. wood is softer than coal b. wood does not rust c. wood can rot d. wood can burn
Answers: 1
You know the right answer?
At 100oC the equilibrium constant for the reaction below has the value of Keq = 2.19 x 10−10. COCl2(...
Questions

Mathematics, 13.06.2021 07:40


Spanish, 13.06.2021 07:40


Geography, 13.06.2021 07:40

Mathematics, 13.06.2021 07:40

Mathematics, 13.06.2021 07:40


Mathematics, 13.06.2021 07:40





Physics, 13.06.2021 07:50

Chemistry, 13.06.2021 07:50

Mathematics, 13.06.2021 07:50

Chemistry, 13.06.2021 07:50



Mathematics, 13.06.2021 07:50