Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
An exothermic reaction is conducted in an insulated calorimeter filled with water. the calorimeter is then sealed so that there is no heat exchanged between the contents of the container and the surrounding air. which of the following statements is true about the reaction?
Answers: 1

Chemistry, 22.06.2019 04:30
Long term exposure to waves can cause sunburns and skin cancer. a) visible b) infrared c) gamma rays d) ultraviole
Answers: 1

Chemistry, 22.06.2019 11:30
Voltaic cells produce a positive overall charge. what does this indicate? a. the reaction is likely to be endothermic. b. the reaction is spontaneous. c. the reaction is not likely to occur. d. the reaction is not spontaneous.
Answers: 3

Chemistry, 22.06.2019 21:00
As we move from left to right across the periodic table, what is the general trend? a) atomic radii increase. b) electronegavitiy decreases. c) nuclear shielding increases. d) metallic character decreases.
Answers: 1
You know the right answer?
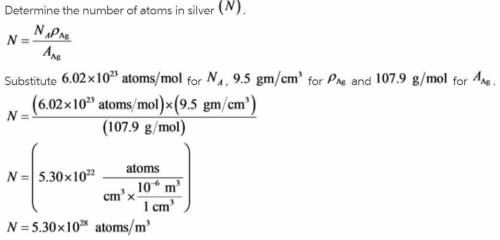
Alculate the energy for vacancy formation in silver, given that the equilibrium number of vacancies...
Questions


Mathematics, 28.10.2020 21:10



Biology, 28.10.2020 21:10




Mathematics, 28.10.2020 21:10

Mathematics, 28.10.2020 21:10


Mathematics, 28.10.2020 21:10


Social Studies, 28.10.2020 21:10



History, 28.10.2020 21:10


History, 28.10.2020 21:10

Mathematics, 28.10.2020 21:10