Chemistry, 20.02.2020 03:45 micahatwood03
Imagine that 27.0 g of C2H2(g) dissolves in 1.00 L of liquid acetone at 1.00 atm pressure. If the partial pressure of C2H2(g) is increased to 12.0 atm, what is its solubility in acetone?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 3

Chemistry, 22.06.2019 08:00
An observation that requires measurement is called quantitative observable or qualitative
Answers: 1


Chemistry, 22.06.2019 12:00
Why are people not able to skip a dive to the deepest part of the ocean
Answers: 1
You know the right answer?
Imagine that 27.0 g of C2H2(g) dissolves in 1.00 L of liquid acetone at 1.00 atm pressure. If the pa...
Questions



Mathematics, 31.07.2019 01:30


English, 31.07.2019 01:30


Social Studies, 31.07.2019 01:30

Mathematics, 31.07.2019 01:30


Mathematics, 31.07.2019 01:30

English, 31.07.2019 01:30

History, 31.07.2019 01:30

Social Studies, 31.07.2019 01:30

Mathematics, 31.07.2019 01:30

History, 31.07.2019 01:30





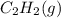 at 12.0 atm is
at 12.0 atm is 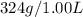
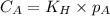
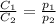
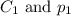 are the initial concentration and partial pressure of
are the initial concentration and partial pressure of 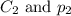 are the final concentration and partial pressure of
are the final concentration and partial pressure of