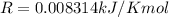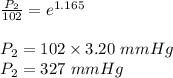Chemistry, 20.02.2020 01:51 olaffm9799
The vapor pressure of ethanol is 1.00 × 102 mmHg at 34.90°C. What is its vapor pressure at 60.21°C? (ΔHvap for ethanol is 39.3 kJ/mol.)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 15:20
Which description best characterizes the motion of particles in a solid?
Answers: 2

Chemistry, 23.06.2019 00:00
Mercury turns to a vapor at 629.88 k. how much heat is lost when 75.0 g of mercury vapor at 650 k condenses to a liquid at 297 k?
Answers: 1

Chemistry, 23.06.2019 01:30
The biomedical technique in which a part of the brain is destroyed with electric current is known as a. electroconvulsive therapy b. prefrontal lobotomy c. bilateral cingulotomy d. tardive dyskinesia
Answers: 2

Chemistry, 23.06.2019 03:00
Asample of sea water contains 6.28g of sodium chloride per litre of solution. how many milligrams of sodium chloride would be contained in 15.0ml of this solution?
Answers: 3
You know the right answer?
The vapor pressure of ethanol is 1.00 × 102 mmHg at 34.90°C. What is its vapor pressure at 60.21°C?...
Questions






Geography, 31.03.2020 00:22


Mathematics, 31.03.2020 00:22

Physics, 31.03.2020 00:22

History, 31.03.2020 00:22





Biology, 31.03.2020 00:22

Mathematics, 31.03.2020 00:22

History, 31.03.2020 00:22



Physics, 31.03.2020 00:22

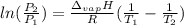
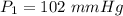
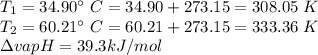
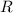 is the Universal Gas Constant.
is the Universal Gas Constant.