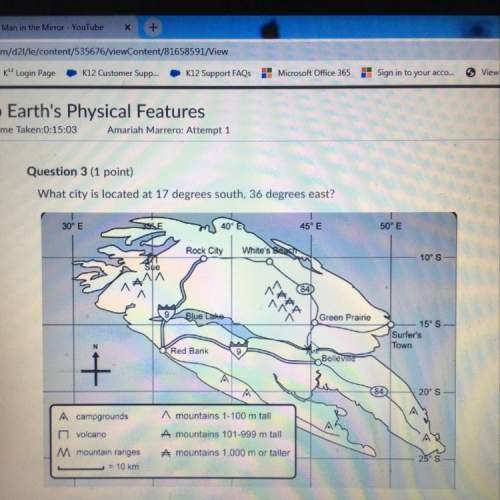
Propose a plausible two-step mechanism for the reaction given below with the steps provided. A+2B → C + E (slow) D + B → E (fast) 28 → C + E (slow) A + E → D (fast) A+B→C+D(slow) A+B→C+E(slow) A+B→C+E(fast) A+B→C+D(fast) D→C+E(fast) Overall Reaction: A+2B → C + E Experimentally Determined Rate Law rate = MA]

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:10
Think about how you can use le chatelier’s principle to find possible solutions to the design problem. describe at least two ways to increase the yield (amount) of ammonia based on this principle.
Answers: 2

Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1

Chemistry, 22.06.2019 12:20
Consider the reaction of a(g) + b(g) + c(g) => d(g) for which the following data were obtained: experiment initial [a], mol/l initial [b], mol/l initial [c], mol/l initial rate, mol/l.s 1 0.0500 0.0500 0.0100 6.25 x 10^-3 2 0.100 0.0500 0.0100 2.50 x 10^-2 3 0.100 0.100 0.0100 1.00 x 10^-1 4 0.0500 0.0500 0.0200 6.25 x 10^-3 what is the rate law for the reaction?
Answers: 3

Chemistry, 22.06.2019 13:00
Is 9 correct? and can someone me with 10? it’s due tomorrow, you
Answers: 1
You know the right answer?
Propose a plausible two-step mechanism for the reaction given below with the steps provided. A+2B →...
Questions


Biology, 13.09.2019 03:30


History, 13.09.2019 03:30