
Chemistry, 19.02.2020 18:42 rhineharttori
Given the balanced equation for the reaction of butane and oxygen: 2C4H10 + 13O2 > 8CO2 + 10H2O + energy How many moles of carbon dioxide are produced when 5.0 moles of butane react completely? * 5.0 mol 10. mol 20. mol 40. mol

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Used the balanced equation 2h2+ o2 - -> 2h2o. if you have 7.2 grams of o2 , how many grams of h2o can you produce ?
Answers: 2

Chemistry, 22.06.2019 05:50
What are transitions between a liquid and a solid called? identify which way they are transitioning
Answers: 2

Chemistry, 22.06.2019 08:40
What is the value of keq for the reaction expressed in scientific notation?
Answers: 1

Chemistry, 22.06.2019 14:20
7. in the cycle, a virus integrates its dna into the host's dna, and its dna is replicated when the host dna is replicated. a. infectious b. retroviral c. lysogenic d.lytic
Answers: 1
You know the right answer?
Given the balanced equation for the reaction of butane and oxygen: 2C4H10 + 13O2 > 8CO2 + 10H2O +...
Questions

History, 15.10.2019 12:30




Mathematics, 15.10.2019 12:30


Arts, 15.10.2019 12:30


Biology, 15.10.2019 12:30

History, 15.10.2019 12:30

English, 15.10.2019 12:30


Chemistry, 15.10.2019 12:30


Computers and Technology, 15.10.2019 12:30

Mathematics, 15.10.2019 12:30

Biology, 15.10.2019 12:30



Geography, 15.10.2019 12:30

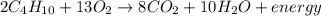
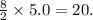 moles of carbon dioxide
moles of carbon dioxide

