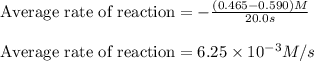Chemistry, 19.02.2020 17:28 kiaraphilman2956
In the first 22.0 s of this reaction, the concentration of HBr dropped from 0.590 M to 0.465 M . Calculate the average rate of the reaction in this time interval.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 14:40
What type of solution is formed if 10 g of kclo3 are dissolved in 100g of water at 30
Answers: 2


Chemistry, 22.06.2019 18:30
Which rate indicates the number of children that would be born per woman if she were to live to the end of her child bearing years
Answers: 2

Chemistry, 22.06.2019 22:40
Percent ionization for a weak acid (ha) is determined by the following formula: percent ionization=[ha] ionized[ha] initial×100%for strong acids, ionization is nearly complete (100%) at most concentrations. however, for weak acids, the percent ionization changes significantly with concentration. the more diluted the acid is, the greater percent ionization.a certain weak acid, ha, has a ka value of 9.4×10? 7.part acalculate the percent ionization of ha in a 0.10 m solution.part bcalculate the percent ionization of ha in a 0.010 m solution
Answers: 1
You know the right answer?
In the first 22.0 s of this reaction, the concentration of HBr dropped from 0.590 M to 0.465 M . Cal...
Questions

Biology, 27.09.2019 18:50

English, 27.09.2019 18:50

Health, 27.09.2019 18:50

Mathematics, 27.09.2019 18:50




Mathematics, 27.09.2019 18:50



History, 27.09.2019 18:50

History, 27.09.2019 18:50

Mathematics, 27.09.2019 18:50




Mathematics, 27.09.2019 18:50


Biology, 27.09.2019 18:50

Social Studies, 27.09.2019 18:50

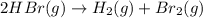
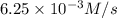
![\text{Average rate of disappearance of HBr}=-\frac{\Delta [HBr]}{\Delta t}](/tpl/images/0515/7057/16c66.png)
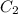 = final concentration of HBr = 0.465 M
= final concentration of HBr = 0.465 M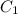 = initial concentration of HBr = 0.590 M
= initial concentration of HBr = 0.590 M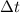 = change in time = 22.0 s
= change in time = 22.0 s