
Chemistry, 19.02.2020 05:52 animerocks07
Determining reaction order : Rate Laws(Chemistry)
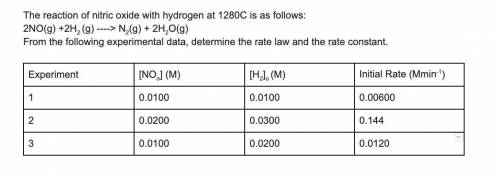
The reaction of nitric oxide with hydrogen at 1280C is as follows:
2NO(g) +2H2 (g) -> N2(g) + 2H2O(g)
From the following experimental data, determine the rate law and the rate constant.
30 POINTS!

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium chlorate
Answers: 3

Chemistry, 22.06.2019 21:00
Once similarity and one difference between a mixture of elements and a mixture of compounds
Answers: 3

Chemistry, 23.06.2019 08:40
A20 liter cylinder of helium at a pressure of 150 atm and a temperature of 27°c is used to fill a balloon at 1.00 atm and 37°c. what is the volume of the balloon? a. 0.14 liters b. 3000 liters c. 2900 liters d. 2400 liters e. 3100 liters
Answers: 1
You know the right answer?
Determining reaction order : Rate Laws(Chemistry)
The reaction of nitric oxide with hydrogen...
The reaction of nitric oxide with hydrogen...
Questions

Mathematics, 25.09.2020 20:01



English, 25.09.2020 20:01

Mathematics, 25.09.2020 20:01

English, 25.09.2020 20:01


History, 25.09.2020 20:01

English, 25.09.2020 20:01

English, 25.09.2020 20:01




Biology, 25.09.2020 20:01

Mathematics, 25.09.2020 20:01


Spanish, 25.09.2020 20:01

Mathematics, 25.09.2020 20:01

Mathematics, 25.09.2020 20:01

Mathematics, 25.09.2020 20:01

![R=k[NO]3[H_2]^1](/tpl/images/0515/4935/da8f4.png)
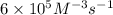
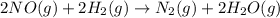
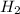 in rate law be x and y .
in rate law be x and y .![R=k[NO]^[H_2]^y](/tpl/images/0515/4935/cf38a.png)
![[NO]=0.0100 M, [H_2]=0.0100 M](/tpl/images/0515/4935/aa668.png)
![0.00600 M/s=k[0.0100 M]^x[0.0100 M]^y](/tpl/images/0515/4935/a6007.png) ..[1]
..[1]![[NO]=0.0200 M, [H_2]=0.0300 M](/tpl/images/0515/4935/ba703.png)
![0.144 M/s=k[0.0200 M]^x[0.0300 M]^y](/tpl/images/0515/4935/0042d.png) ..[2]
..[2]![[NO]=0.0100 M, [H_2]=0.0200 M](/tpl/images/0515/4935/ba63c.png)
![0.0120 M/s=k[0.0100 M]^x[0.0200 M]^y](/tpl/images/0515/4935/9f0d9.png) ..[3]
..[3]![\frac{0.00600 M/s}{0.0120 M/s}=\frac{k[0.0100 M]^x\times [0.0100 M]^y}{k[0.0100 M]^x\times [0.0200 M]^y}](/tpl/images/0515/4935/1b196.png)
![\frac{0.00600 M/s}{0.144 M/s}=\frac{k[0.0100 M]^x\times [0.0100 M]^1}{k[0.0200 M]^x\times [0.0300 M]^1}](/tpl/images/0515/4935/e9761.png)
![0.00600 M/s=k[0.0100 M]^3[0.0100 M]^1](/tpl/images/0515/4935/2cbdd.png) ..[1]
..[1]![k=\frac{0.00600 M/s}{[0.0100 M]^3[0.0100 M]^1}=6\times 10^5 M^{-3}s^{-1}](/tpl/images/0515/4935/88d8d.png)


