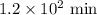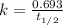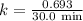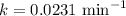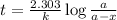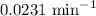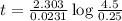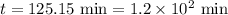Chemistry, 19.02.2020 05:17 caitlynnpatton1208
The compound Xe(CF3)2 decomposes in a first-order reaction to elemental Xe with a half-life of 30.0 min. If you place 4.5 mg of Xe(CF3)2 in a flask, calculate how long you must wait until only 0.25 mg of Xe(CF3)2 remains?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Explain how the integumentary system plays a crucial role in the ability to maintain homeoestasis
Answers: 1

Chemistry, 22.06.2019 13:30
If the concentration of phosphate in the cytosol is 2.0 mm and the concentration of phosphate in the surrounding fluid is 0.1 mm, how could the cell increase the concentration of phosphate in the cytosol? a) passive transportb) diffusionc) active transportd) osmosise) facilitated diffusion
Answers: 3

Chemistry, 22.06.2019 17:30
Upon decomposition, one sample of magnesium fluoride produced 1.65 kg of magnesium and 2.56 kg of fluorine. a second sample produced 1.32 kg of magnesium. part a how much fluorine (in grams) did the second sample produce?
Answers: 2

Chemistry, 22.06.2019 21:50
What is a main difference between a mixture and a pure substance? a mixture is only a liquid, but a pure substance can be in any state.a mixture looks the same throughout, but a pure substance does not.1 a mixture can vary in composition, but a pure substance has a set composlo a mixture can be made up of a single compound, but a pure substance car
Answers: 2
You know the right answer?
The compound Xe(CF3)2 decomposes in a first-order reaction to elemental Xe with a half-life of 30.0...
Questions



Chemistry, 26.10.2019 23:43

Biology, 26.10.2019 23:43


Biology, 26.10.2019 23:43

Geography, 26.10.2019 23:43


Chemistry, 26.10.2019 23:43


Biology, 26.10.2019 23:43

Arts, 26.10.2019 23:43

Mathematics, 26.10.2019 23:43


Social Studies, 26.10.2019 23:43

Mathematics, 26.10.2019 23:43


Mathematics, 26.10.2019 23:43


History, 26.10.2019 23:43