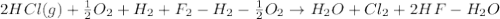Chemistry, 19.02.2020 02:23 Javanese5987
From the following enthalpies of reaction,
4 HCl (g) + O2 (g) → 2 H2O (l) + 2 Cl2 (g) ∆H = -202.4 kJ/mol 1/2 H2 (g) + ½ F2 (g) → HF (l) ∆H = -600.0 kJ/mol H2 (g) + ½ O2 (g) → H2O (l) ∆H = -285.8 kJ/mol
Calculate ∆Hrxn for 2 HCl (g) + F2 (g) → 2 HF (l) + Cl2 (g) Just input a number.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:20
What is the strongest intermolecular force between an nacl unit and an h2o molecule together in a solution? covalent bonding dipole-dipole force hydrogen bonding ion-dipole force
Answers: 1


Chemistry, 22.06.2019 09:20
Explain that newton first law,second law and third law of motion?
Answers: 2

Chemistry, 22.06.2019 12:00
Give the set of reactants (including an alkyl halide and a nucleophile) that could be used to synthesize the following ether: draw the molecules on the canvas by choosing buttons from the tools (for bonds and charges), atoms, and templates toolbars, including charges where needed. ch3ch2och2ch2chch3 | ch3
Answers: 1
You know the right answer?
From the following enthalpies of reaction,
4 HCl (g) + O2 (g) → 2 H2O (l) + 2 Cl2 (g) ∆H = -2...
4 HCl (g) + O2 (g) → 2 H2O (l) + 2 Cl2 (g) ∆H = -2...
Questions

Biology, 25.07.2019 20:00

English, 25.07.2019 20:00

Social Studies, 25.07.2019 20:00

Social Studies, 25.07.2019 20:00


Social Studies, 25.07.2019 20:00


Mathematics, 25.07.2019 20:00




Social Studies, 25.07.2019 20:00



Mathematics, 25.07.2019 20:00




Chemistry, 25.07.2019 20:00

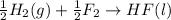 ΔH=-600.0 KJ/mol
ΔH=-600.0 KJ/mol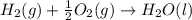 ΔH= -285.8 KJ/mol
ΔH= -285.8 KJ/mol