
Chemistry, 19.02.2020 02:45 blayneaafedt
Chromium has an atomic mass of 51.9961 u and consists of four isotopes, 50 Cr , 52 Cr , 53 Cr , and 54 Cr . The 52 Cr isotope has a natural abundance of 83.79 % and an atomic mass of 51.9405 u. The 54 Cr isotope has a natural abundance of 2.37 % and an atomic mass of 53.9389 u. The natural abundances of the 50 Cr and 53 Cr isotopes exist in a ratio of 0.4579 : 1 , and the 50 Cr isotope has an atomic mass of 49.9460 u. Determine the atomic mass of the 53 Cr isotope. atomic mass of 53 Cr:.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:30
The atomic radius of sodium is 186 pm and of chlorine is 100 pm. the ionic radius for na+ is 102 pm and for cl– is 181 pm. in going from na to cl in period 3, why does the atomic radius decrease while the ionic radius increases? a. the inner electrons in the sodium cation shield its valence electrons more effectively than the inner electrons in the chloride anion do. b. the inner electrons shield the valence electrons more effectively in the chlorine atom than in the chloride anion. c. the outermost electrons in chloride experience a smaller effective nuclear charge than those in the sodium cation do. d. the outermost electrons in chloride experience a larger effective nuclear charge than those in the sodium cation do. e. monatomic ions are bigger than the atoms from which they are formed.
Answers: 2


Chemistry, 22.06.2019 08:30
For each of the compounds below, show that the charges on the ions add up to zero. a. kbr b. cao c. li(2)o d. cacl(2) e. alcl(3)
Answers: 2

You know the right answer?
Chromium has an atomic mass of 51.9961 u and consists of four isotopes, 50 Cr , 52 Cr , 53 Cr , and...
Questions

Mathematics, 24.10.2020 03:20

History, 24.10.2020 03:20



Geography, 24.10.2020 03:20

Mathematics, 24.10.2020 03:20

English, 24.10.2020 03:20

Mathematics, 24.10.2020 03:20

Mathematics, 24.10.2020 03:20

Mathematics, 24.10.2020 03:20

Spanish, 24.10.2020 03:20


Mathematics, 24.10.2020 03:20


Mathematics, 24.10.2020 03:20




Mathematics, 24.10.2020 03:20

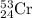 isotope is 52.8367 amu
isotope is 52.8367 amu![_{24}^{50}\text{Cr}\text{ and }_{24}^{53}\textrm{Cr}\text{ isotopes}=[100-(83.79+2.37)]=13.84\%](/tpl/images/0515/1858/e24f2.png)
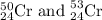 isotopes = 0.4579 : 1
isotopes = 0.4579 : 1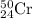 isotope =
isotope = 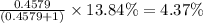
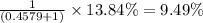
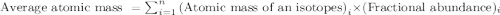 .....(1)
.....(1)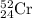 isotope:
isotope: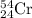 isotope:
isotope:![51.9961=[(49.9460\times 0.0437)+(51.9405\times 0.8379)+(x\times 0.0949)+(53.9389\times 0.0237)]\\\\x=52.8367amu](/tpl/images/0515/1858/822e6.png)


