
Consider the following system at equilibrium: 2A(aq)+2B(aq)⇌5C(aq) Classify each of the following actions by whether it causes a leftward shift, a rightward shift, or no shift in the direction of the net reaction. a. increase (b)
b. increase(a)
c. increase(c)
d. decrease(a)
e. decrease(b)
f. decrease(c)
g. double(a) and reduce (b) to one half
h. double both (b) and (c)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
This active feature of earth's crust in building mountain ranges as well as islands. this feature is a a) cavern. b) earthquake. c) mountain. d) volcano.
Answers: 2

Chemistry, 22.06.2019 04:30
There is a single path for electrons. the current decreases when additional resistors are added. the current will be the same in each resistor. these statements best describe a(n) circuit.
Answers: 3

Chemistry, 22.06.2019 23:00
What is the solubility-product constant of barium sulfate, baso4, if a saturated solution is 1.03 ´ 10-5 m?
Answers: 3

Chemistry, 23.06.2019 00:20
How many lone pairs of electrons are on the central atom of no3- and what is the molecular shape? one, trigonal planar zero, trigonal pyramidal zero, trigonal planar one, tetrahedral one, trigonal pyramidal
Answers: 1
You know the right answer?
Consider the following system at equilibrium: 2A(aq)+2B(aq)⇌5C(aq) Classify each of the following ac...
Questions



Geography, 18.10.2020 08:01




English, 18.10.2020 08:01

Mathematics, 18.10.2020 08:01


Mathematics, 18.10.2020 08:01

Mathematics, 18.10.2020 08:01

Mathematics, 18.10.2020 08:01


Chemistry, 18.10.2020 08:01


Spanish, 18.10.2020 08:01

Physics, 18.10.2020 08:01

Social Studies, 18.10.2020 08:01


English, 18.10.2020 08:01

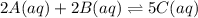
![K_{c}=\frac{[C(aq)]^5}{[A(aq)]^2\cdot [B(aq)]^2}](/tpl/images/0515/1184/e02e8.png)
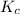 is the same, meaning that an increase in the concentration of the species B must cause a rightward shift to increase the concentration of the species C, such that the ratio expressed by the equilibrium constant remains unchanged.
is the same, meaning that an increase in the concentration of the species B must cause a rightward shift to increase the concentration of the species C, such that the ratio expressed by the equilibrium constant remains unchanged.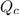 to compare with the equilibrium constant
to compare with the equilibrium constant ![Q{c}=\frac{[C(aq)]^5}{[A(aq)]^2\cdot [B(aq)]^2}](/tpl/images/0515/1184/564e9.png)


