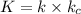Chemistry, 19.02.2020 01:25 NetherisIsTheQueen
The following mechanism has been proposed for the gas phase reaction of nitrogen monoxide with bromine.
step 1 fast: NO Br2 NOBr2
step 2 slow: NOBr2 NO 2 NOBr
(1) What is the equation for the overall reaction
(2) Enter the formula of any species that acts as a reaction intermediate?
(3) Complete the rate law for the overall reaction that is consistent with this mechanism.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Which term best describes the form sound takes as it travels away from a drum (a- gas)(b-music) ( c-waves) (d-particles
Answers: 3

Chemistry, 21.06.2019 22:30
Monkeys and bats have similar bone structure in their forelimbs. however, monkeys have longer forelimbs to use for climbing and swinging in trees. bats have shorter forelimbs to use for flight. which term best describes how monkey and bat forelimbs are related to each other? a. homologous b. embryonic c. analogous d. vestigial
Answers: 1

Chemistry, 22.06.2019 03:50
Which of the following statements about acidic water is true? a. acid has no effect on the h,o molecules. b. the solution contains a larger number of oh ions than h,o ions. c. the solution contains a larger number of h,o ions than qh ions. d. the solution contains an equal number of h,o ions and oh ions. none of the above e.
Answers: 1

You know the right answer?
The following mechanism has been proposed for the gas phase reaction of nitrogen monoxide with bromi...
Questions

Mathematics, 12.01.2021 23:00

Mathematics, 12.01.2021 23:00

Mathematics, 12.01.2021 23:00

Mathematics, 12.01.2021 23:00

Mathematics, 12.01.2021 23:00






History, 12.01.2021 23:00

Business, 12.01.2021 23:00


Chemistry, 12.01.2021 23:00

Mathematics, 12.01.2021 23:00


Mathematics, 12.01.2021 23:00


Mathematics, 12.01.2021 23:00

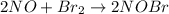
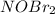 .
.![R=K[NO]^2[Br]](/tpl/images/0515/0512/945c3.png)
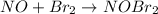 ..[1]
..[1]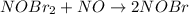 ...[2]
...[2]![R=k[NOBr_2][NO]](/tpl/images/0515/0512/7dc1f.png) ..[3]
..[3]![K_c=\frac{[NOBr_2]}{[NO][Br_2]}](/tpl/images/0515/0512/8d77b.png)
![[NOBr_2]=K_c\times [NO][Br_2]](/tpl/images/0515/0512/df1dc.png)
![[NOBr_2]](/tpl/images/0515/0512/86582.png) rate expression [3]:
rate expression [3]:![R=k\times k_c[NO][NO][NO]=K[NO]^2[Br]](/tpl/images/0515/0512/f99df.png)