
Chemistry, 19.02.2020 00:56 rouchedavisin4
For a reaction with ΔH° = −9 kJ/mol, decide if the following statement is true. If it is false, choose the statement that makes it true. Select the single best answer. The reaction is exothermic. (Assume the reaction has a small entropy term compared to the enthalpy term) a. The statement is false. b. The reaction does not occur. c. The statement is true. d. The statement is false. e. The reaction is endergonic. f. The statement is false. g. The reaction is endothermic.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 09:30
Which formula can be used to calculate the molar mass of hydrogen peroxide
Answers: 1


You know the right answer?
For a reaction with ΔH° = −9 kJ/mol, decide if the following statement is true. If it is false, choo...
Questions


Computers and Technology, 06.11.2020 01:00


Biology, 06.11.2020 01:00

Mathematics, 06.11.2020 01:00

Health, 06.11.2020 01:00

Mathematics, 06.11.2020 01:00

English, 06.11.2020 01:00

English, 06.11.2020 01:00

Arts, 06.11.2020 01:00

History, 06.11.2020 01:00

History, 06.11.2020 01:00

Mathematics, 06.11.2020 01:00


English, 06.11.2020 01:00

History, 06.11.2020 01:00

Mathematics, 06.11.2020 01:00


Spanish, 06.11.2020 01:00

History, 06.11.2020 01:00

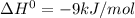 , the heat is released and is exothermic.Thus the statement that the reaction is exothermic is true.
, the heat is released and is exothermic.Thus the statement that the reaction is exothermic is true.

